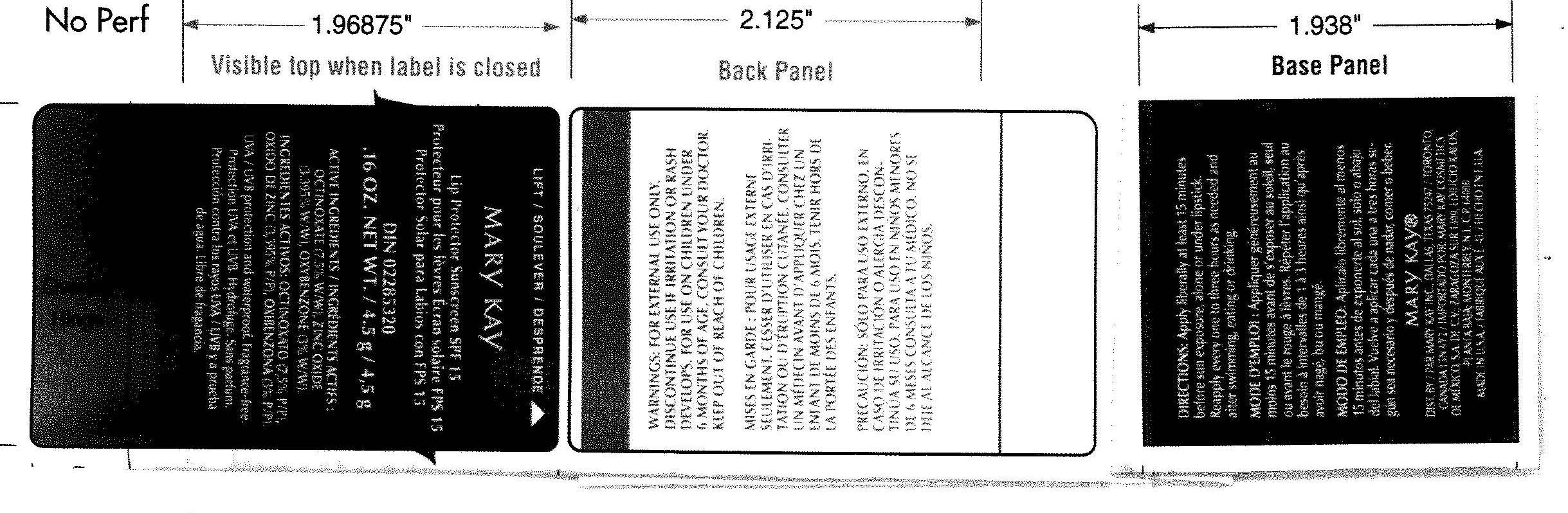
LIP PROTECTOR SUNSCREEN SPF15 LIP PROTECTOR SUNSCREEN SPF15
-
octinoxate,
oxybenzone and
zinc oxide lipstick
Mary Kay INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DIRECTION: Apply liberally at least 15 minutes before sun exposure, alone or under lipstick. Reapply every one to three hours as needed and after swimming, eating or drinking .3
WARNING : For external use only. Discontinue use if irritation or rash develops. For use under 6 month of age , consult your doctor. Keep out reach of children.
PETROLATUM , C12-15 ALKYL BENZOATE , PENTAETYTHRITYL TETRAISOSTEARATE , OZOKERITE , CERESIN , CETYL ALCOHOL , TOCOPHERYL ACETATE, POLYHYDROXYSTERAIC ACID,TOCOPEROL , GLYCINE SOJA (SOYBEAN) OIL , TRIETHOXYCAPRYLRLSILANE , YELLOW 5 LAKE , YELLOW 6 LAKE
| LIP PROTECTOR SUNSCREEN SPF15
LIP PROTECTOR SUNSCREEN SPF15
octinoxate lipstick |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 03/01/2007 | |
| Labeler - Mary Kay INC. (103978839) |
Revised: 02/2011 Mary Kay INC.