darvon-n (propoxyphene napsylate) tablet, film coated
[Xanodyne]
CIV
Rx only
DESCRIPTION
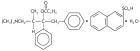
Darvon-N® (Propoxyphene Napsylate, USP) is an odorless, white crystalline powder with a bitter taste. It is very slightly soluble in water and soluble in methanol, ethanol, chloroform, and acetone. Chemically, it is (αS,1R)-α-[2-(Dimethylamino)-1-methylethyl]-α-phenylphenethyl propionate compound with 2-naphthalenesulfonic acid (1:1) monohydrate, which can be represented by the accompanying structural formula. Its molecular weight is 565.72.
Propoxyphene napsylate differs from propoxyphene hydrochloride in that it allows more stable liquid dosage forms and tablet formulations. Because of differences in molecular weight, a dose of 100 mg (176.8 μmol) of propoxyphene napsylate is required to supply an amount of propoxyphene equivalent to that present in 65 mg (172.9 μmol) of propoxyphene hydrochloride.
Each tablet of Darvon-N® contains 100 mg (176.8 μmol) propoxyphene napsylate. The tablet also contains cellulose, cornstarch, iron oxides, lactose, magnesium stearate, silicon dioxide, stearic acid, and titanium dioxide.
CLINICAL PHARMACOLOGY
Propoxyphene is a centrally acting narcotic analgesic agent. Equimolar doses of propoxyphene hydrochloride or napsylate provide similar plasma concentrations. Following administration of 65, 130, or 195 mg of propoxyphene hydrochloride, the bioavailability of propoxyphene is equivalent to that of 100, 200, or 300 mg respectively of propoxyphene napsylate. Peak plasma concentrations of propoxyphene are reached in 2 to 2 1/2 hours. After a 100-mg oral dose of propoxyphene napsylate, peak plasma levels of 0.05 to 0.1 μg/mL are achieved. As shown in Figure 1, the napsylate salt tends to be absorbed more slowly than the hydrochloride. At or near therapeutic doses, this difference is small when compared with that among subjects and among doses.
Figure 1. Mean plasma concentrations of propoxyphene in 8 human subjects following oral administration of 65 and 130 mg of the hydrochloride salt and 100 and 200 mg of the napsylate salt and in 7 given 195 mg of the hydrochloride and 300 mg of the napsylate salt

Figure 1
Because of this several hundredfold difference in solubility, the absorption rate of very large doses of the napsylate salt is significantly lower than that of equimolar doses of the hydrochloride.
Repeated doses of propoxyphene at 6-hour intervals lead to increasing plasma concentrations, with a plateau after the ninth dose at 48 hours.
Propoxyphene is metabolized in the liver to yield norpropoxyphene. Propoxyphene has a half-life of 6 to 12 hours, whereas that of norpropoxyphene is 30 to 36 hours.
Norpropoxyphene has substantially less central-nervous-system-depressant effect than propoxyphene but a greater local anesthetic effect, which is similar to that of amitriptyline and antiarrhythmic agents, such as lidocaine and quinidine.
In animal studies in which propoxyphene and norpropoxyphene were continuously infused in large amounts, intracardiac conduction time (PR and QRS intervals) was prolonged. Any intracardiac conduction delay attributable to high concentrations of norpropoxyphene may be of relatively long duration.
ACTIONS
Propoxyphene is a mild narcotic analgesic structurally related to methadone. The potency of propoxyphene napsylate is from two thirds to equal that of codeine.
INDICATION
For the relief of mild to moderate pain
CONTRAINDICATION
Hypersensitivity to propoxyphene.
WARNINGS
- Do not prescribe propoxyphene for patients who are suicidal or addiction-prone.
- Prescribe propoxyphene with caution for patients taking tranquilizers or antidepressant drugs and patients who use alcohol in excess.
- Tell your patients not to exceed the recommended dose and to limit their intake of alcohol.
Propoxyphene products in excessive doses, either alone or in combination with other CNS depressants, including alcohol, are a major cause of drug-related deaths. Fatalities within the first hour of overdosage are not uncommon. In a survey of deaths due to overdosage conducted in 1975, in approximately 20% of the fatal cases, death occurred within the first hour (5% occurred within 15 minutes). Propoxyphene should not be taken in doses higher than those recommended by the physician. The judicious prescribing of propoxyphene is essential to the safe use of this drug. With patients who are depressed or suicidal, consideration should be given to the use of non-narcotic analgesics. Patients should be cautioned about the concomitant use of propoxyphene products and alcohol because of potentially serious CNS-additive effects of these agents. Because of its added depressant effects, propoxyphene should be prescribed with caution for those patients whose medical condition requires the concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Patients should be advised of the additive depressant effects of these combinations.
Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation or attempts as well as histories of misuse of tranquilizers, alcohol, and other CNS-active drugs. Some deaths have occurred as a consequence of the accidental ingestion of excessive quantities of propoxyphene alone or in combination with other drugs. Patients taking propoxyphene should be warned not to exceed the dosage recommended by the physician.
Drug Dependence—Propoxyphene, when taken in higher-than-recommended doses over long periods of time, can produce drug dependence characterized by psychic dependence and, less frequently, physical dependence and tolerance. Propoxyphene will only partially suppress the withdrawal syndrome in individuals physically dependent on morphine or other narcotics. The abuse liability of propoxyphene is qualitatively similar to that of codeine although quantitatively less, and propoxyphene should be prescribed with the same degree of caution appropriate to the use of codeine.
Usage in Ambulatory Patients—Propoxyphene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
PRECAUTIONS
General—Propoxyphene should be administered with caution to patients with hepatic or renal impairment since higher serum concentrations or delayed elimination may occur.
Drug Interactions—The CNS-depressant effect of propoxyphene is additive with that of other CNS depressants, including alcohol.
As is the case with many medicinal agents, propoxyphene may slow the metabolism of a concomitantly administered drug. Should this occur, the higher serum concentrations of that drug may result in increased pharmacologic or adverse effects of that drug. Such occurrences have been reported when propoxyphene was administered to patients on antidepressants, anticonvulsants, or warfarin-like drugs. Severe neurologic signs, including coma, have occurred with concurrent use of carbamazepine.
Usage in Pregnancy—Safe use in pregnancy has not been established relative to possible adverse effects on fetal development. Instances of withdrawal symptoms in the neonate have been reported following usage during pregnancy. Therefore, propoxyphene should not be used in pregnant women unless, in the judgment of the physician, the potential benefits outweigh the possible hazards.
Usage in Nursing Mothers—Low levels of propoxyphene have been detected in human milk. In postpartum studies involving nursing mothers who were given propoxyphene, no adverse effects were noted in infants receiving mother's milk.
Usage in Pediatric Patients—Safety and effectiveness in pediatric patients have not been established.
Usage in the Elderly—The rate of propoxyphene metabolism may be reduced in some patients. Increased dosing interval should be considered.
A Patient Information Sheet is available for this product. See text following “How Supplied” section below.
ADVERSE REACTIONS
In a survey conducted in hospitalized patients, less than 1% of patients taking propoxyphene hydrochloride at recommended doses experienced side effects. The most frequently reported were dizziness, sedation, nausea, and vomiting. Some of these adverse reactions may be alleviated if the patient lies down.
Other adverse reactions include constipation, abdominal pain, skin rashes, lightheadedness, headache, weakness, euphoria, dysphoria, hallucinations, and minor visual disturbances.
Propoxyphene therapy has been associated with abnormal liver function tests and, more rarely, with instances of reversible jaundice (including cholestatic jaundice).
Subacute painful myopathy has occurred following chronic propoxyphene overdosage.
DOSAGE AND ADMINISTRATION
Darvon-N® is given orally. The usual dosage is 100 mg propoxyphene napsylate every 4 hours as needed for pain. The maximum recommended dose of propoxyphene napsylate is 600 mg per day.
Consideration should be given to a reduced total daily dosage in patients with hepatic or renal impairment.
MANAGEMENT OF OVERDOSAGE
In all cases of suspected overdosage, call your regional Poison Control Center to obtain the most up-to-date information about the treatment of overdose. This recommendation is made because, in general, information regarding the treatment of overdosage may change more rapidly than do package inserts.
Initial consideration should be given to the management of the CNS effects of propoxyphene overdosage. Resuscitative measures should be initiated promptly.
Symptoms of Propoxyphene Overdosage—The manifestations of acute overdosage with propoxyphene are those of narcotic overdosage. The patient is usually somnolent but may be stuporous or comatose and convulsing. Respiratory depression is characteristic. The ventilatory rate and/or tidal volume is decreased, which results in cyanosis and hypoxia. Pupils, initially pinpoint, may become dilated as hypoxia increases. Cheyne-Stokes respiration and apnea may occur. Blood pressure and heart rate are usually normal initially, but blood pressure falls and cardiac performance deteriorates, which ultimately results in pulmonary edema and circulatory collapse, unless the respiratory depression is corrected and adequate ventilation is restored promptly. Cardiac arrhythmias and conduction delay may be present. A combined respiratory-metabolic acidosis occurs owing to retained CO2 (hypercapnia) and to lactic acid formed during anaerobic glycolysis. Acidosis may be severe if large amounts of salicylates have also been ingested. Death may occur.
Treatment of Propoxyphene Overdosage—Attention should be directed first to establishing a patent airway and to restoring ventilation. Mechanically assisted ventilation, with or without oxygen, may be required, and positive pressure respiration may be desirable if pulmonary edema is present. The narcotic antagonist naloxone will markedly reduce the degree of respiratory depression, and 0.4 to 2 mg should be administered promptly, preferably intravenously. If the desired degree of counteraction with improvement in respiratory functions is not obtained, naloxone should be repeated at 2- to 3-minute intervals. The duration of action of the antagonist may be brief. If no response is observed after 10 mg of naloxone have been administered, the diagnosis of propoxyphene toxicity should be questioned. Naloxone may also be administered by continuous intravenous infusion.
Treatment of Propoxyphene Overdosage in Pediatric Patients—The usual initial dose of naloxone in pediatric patients is 0.01 mg/kg body weight given intravenously. If this dose does not result in the desired degree of clinical improvement, a subsequent increased dose of 0.1 mg/kg body weight may be administered. If an IV route of administration is not available, naloxone may be administered IM or subcutaneously in divided doses. If necessary, naloxone can be diluted with Sterile Water for Injection.
Blood gases, pH, and electrolytes should be monitored in order that acidosis and any electrolyte disturbance present may be corrected promptly. Acidosis, hypoxia, and generalized CNS depression predispose to the development of cardiac arrhythmias. Ventricular fibrillation or cardiac arrest may occur and necessitate the full complement of cardiopulmonary resuscitation (CPR) measures. Respiratory acidosis rapidly subsides as ventilation is restored and hypercapnia eliminated, but lactic acidosis may require intravenous bicarbonate for prompt correction.
Electrocardiographic monitoring is essential. Prompt correction of hypoxia, acidosis, and electrolyte disturbance (when present) will help prevent these cardiac complications and will increase the effectiveness of agents administered to restore normal cardiac function.
In addition to the use of a narcotic antagonist, the patient may require careful titration with an anticonvulsant to control convulsions. Analeptic drugs (for example, caffeine or amphetamine) should not be used because of their tendency to precipitate convulsions.
General supportive measures, in addition to oxygen, include, when necessary, intravenous fluids, vasopressor-inotropic compounds, and, when infection is likely, anti-infective agents. Gastric lavage may be useful, and activated charcoal can adsorb a significant amount of ingested propoxyphene. Dialysis is of little value in poisoning due to propoxyphene. Efforts should be made to determine whether other agents, such as alcohol, barbiturates, tranquilizers, or other CNS depressants, were also ingested, since these increase CNS depression as well as cause specific toxic effects.
ANIMAL TOXICOLOGY
The acute lethal doses of the hydrochloride and napsylate salts of propoxyphene were determined in 4 species. The results shown in Figure 2 indicate that, on a molar basis, the napsylate salt is less toxic than the hydrochloride. This may be due to the relative insolubility and retarded absorption of propoxyphene napsylate.

Figure 2
Some indication of the relative insolubility and retarded absorption of propoxyphene napsylate was obtained by measuring plasma propoxyphene levels in 2 groups of 4 dogs following oral administration of equimolar doses of the 2 salts. As shown in Figure 3, the peak plasma concentration observed with propoxyphene hydrochloride was much higher than that obtained after administration of the napsylate salt.
Although none of the animals in this experiment died, 3 of the 4 dogs given propoxyphene hydrochloride exhibited convulsive seizures during the time interval corresponding to the peak plasma levels. The 4 animals receiving the napsylate salt were mildly ataxic but not acutely ill.
Figure 3. Plasma propoxyphene concentrations in dogs following large doses of the hydrochloride and napsylate salts

Figure 3
HOW SUPPLIED
Darvon-N® Tablets are available in:
The 100mg tablets are buff colored, elliptical shaped, film coated, and imprinted with the script “DARVON-N 100” on one side of the tablet, using edible black ink. They are available as follows:
| Bottles of 100 | NDC 66479-512-10 |
| Bottles of 500 | NDC 66479-512-50 |
Store at 25°C (77°F); excursions are permitted to 15°- 30°C (59°- 86°F) [see USP Controlled Room Temperature]
The following information, including description of dosage forms and the maximum daily dosage of each, is available to patients receiving Darvon products.
Patient Information Sheet
YOUR PRESCRIPTION FOR A DARVON®(PROPOXYPHENE) PRODUCT
CIV
Summary
Products containing Darvon are used to relieve pain.
LIMIT YOUR INTAKE OF ALCOHOL WHILE TAKING THIS DRUG.
Make sure your doctor knows if you are taking tranquilizers, sleep aids, antidepressants, antihistamines, or any other drugs that make you sleepy. Combining propoxyphene with alcohol or these drugs in excessive doses is dangerous.
Use care while driving a car or using machines until you see how the drug affects you because propoxyphene can make you sleepy. Do not take more of the drug than your doctor prescribed. Dependence has occurred when patients have taken propoxyphene for a long period of time at doses greater than recommended.
The rest of this leaflet gives you more information about propoxyphene. Please read it and keep it for future use.
Uses of Darvon
Products containing Darvon are used for the relief of mild to moderate pain. Products that contain Darvon plus aspirin or acetaminophen are prescribed for the relief of pain or pain associated with fever.
Before Taking Darvon
Make sure your doctor knows if you have ever had an allergic reaction to propoxyphene, aspirin, or acetaminophen. Some forms of propoxyphene products contain aspirin to help relieve the pain. Your doctor should be advised if you have a history of ulcers or if you are taking an anticoagulant (“blood thinner”). The aspirin may irritate the stomach lining and may cause bleeding, particularly if an ulcer is present. Also, bleeding may occur if you are taking an anticoagulant. In a small group of people, aspirin may cause an asthma attack. If you are one of these people, be sure your drug does not contain aspirin.
The effect of propoxyphene in pediatric patients under 12 has not been studied. Therefore, use of the drug in this age group is not recommended.
Also, due to the possible association between aspirin and Reye Syndrome, those propoxyphene products containing aspirin should not be given to children, including teenagers, with chicken pox or flu unless prescribed by a physician. The following propoxyphene product contains aspirin:
Darvon® Compound-65 (Propoxyphene Hydrochloride, Aspirin, and Caffeine, USP)
How to Take Darvon
Follow your doctor's directions exactly. Do not increase the amount you take without your doctor's approval. If you miss a dose of the drug, do not take twice as much the next time.
Pregnancy
Do not take propoxyphene during pregnancy unless your doctor knows you are pregnant and specifically recommends its use. Cases of temporary dependence in the newborn have occurred when the mother has taken propoxyphene consistently in the weeks before delivery. As a general principle, no drug should be taken during pregnancy unless it is clearly necessary.
General Cautions
Heavy use of alcohol with propoxyphene is hazardous and may lead to overdosage symptoms (see “ Overdose” below). THEREFORE, LIMIT YOUR INTAKE OF ALCOHOL WHILE TAKING PROPOXYPHENE.
Combinations of excessive doses of propoxyphene, alcohol, and tranquilizers are dangerous. Make sure your doctor knows if you are taking tranquilizers, sleep aids, antidepressant drugs, antihistamines, or any other drugs that make you sleepy. The use of these drugs with propoxyphene increases their sedative effects and may lead to overdosage symptoms, including death (see “ Overdose” below).
Propoxyphene may cause drowsiness or impair your mental and/or physical abilities; therefore, use caution when driving a vehicle or operating dangerous machinery. DO NOT perform any hazardous task until you have seen your response to this drug.
Propoxyphene may increase the concentration in the body of medications, such as anticoagulants (“blood thinners”), antidepressants, or drugs used for epilepsy. The result may be excessive or adverse effects of these medications. Make sure your doctor knows if you are taking any of these medications.
Dependence
You can become dependent on propoxyphene if you take it in higher than recommended doses over a long period of time. Dependence is a feeling of need for the drug and a feeling that you cannot perform normally without it.
Overdose
An overdose of Darvon, alone or in combination with other drugs, including alcohol, may cause weakness, difficulty in breathing, confusion, anxiety, and more severe drowsiness and dizziness. Extreme overdosage may lead to unconsciousness and death.
If the propoxyphene product contains acetaminophen, the overdosage symptoms include nausea, vomiting, lack of appetite, and abdominal pain. Liver damage may occur.
When the propoxyphene product contains aspirin, symptoms of taking too much of the drug are headache, dizziness, ringing in the ears, difficulty in hearing, dim vision, confusion, drowsiness, sweating, thirst, rapid breathing, nausea, vomiting, and, occasionally, diarrhea.
In any suspected overdosage situation, contact your doctor or nearest hospital emergency room. GET EMERGENCY HELP IMMEDIATELY.
KEEP THIS DRUG AND ALL DRUGS OUT OF THE REACH OF THE PEDIATRIC POPULATION.
Possible Side Effects
When propoxyphene is taken as directed, side effects are infrequent. Among those reported are drowsiness, dizziness, nausea, and vomiting. If these effects occur, it may help if you lie down and rest.
Less frequently reported side effects are constipation, abdominal pain, skin rashes, lightheadedness, headache, weakness, hallucinations, minor visual disturbances, and feelings of elation or discomfort.
If side effects occur and concern you, contact your doctor.
Other Information
The safe and effective use of propoxyphene depends on your taking it exactly as directed. This drug has been prescribed specifically for you and your present condition. Do not give this drug to others who may have similar symptoms. Do not use it for any other reason.
If you would like more information about propoxyphene, ask your doctor or pharmacist. They have a more technical leaflet (professional labeling) you may read.
Darvon, Darvon-N, Darvocet, and Darvocet-N are registered trademarks of
Xanodyne Pharmaceuticals Inc.
© 2006 Xanodyne Pharmaceuticals Inc.
Marketed by:
Xanodyne®
Newport, KY 41071
PC3366B
REV. 02-2006
| Darvon-N (propoxyphene napsylate) | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
Revised: 10/2007Xanodyne