FLUZONE
-
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated),
influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated) and
influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension
FLUZONE HIGH-DOSE
-
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated),
influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated) and
influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension
FLUZONE INTRADERMAL
-
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated),
influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated) and
influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension
Sanofi Pasteur Inc.
----------
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
Fluzone®, Fluzone High-Dose, and Fluzone Intradermal are inactivated influenza virus vaccines indicated for active immunization against influenza disease caused by influenza virus subtypes A and type B contained in the vaccine.
Fluzone is approved for use in persons 6 months of age and older. Fluzone High-Dose is approved for use in persons 65 years of age and older. Fluzone Intradermal is approved for use in persons 18 through 64 years of age.
Approval of Fluzone High-Dose is based on superior immune response relative to Fluzone. Data demonstrating a decrease in influenza disease after vaccination with Fluzone High-Dose relative to Fluzone are not available.
2. DOSAGE AND ADMINISTRATION
2.1. Dosage and Schedule
The dose, schedule, and route of administration for Fluzone, Fluzone High-Dose and Fluzone Intradermal are presented in Table 1, Table 2 and Table 3.
| Vaccination Status and Age | Dose/Route | Schedule |
|---|---|---|
| 6 months through 8 years, previously unvaccinated or vaccinated for the first time last season with only one dose of influenza vaccine | ||
| 6 months through 35 months | 0.25 mL/Intramuscular | 2 doses at least 1 month apart |
| 36 months through 8 years | 0.5 mL/Intramuscular | 2 doses at least 1 month apart |
| 6 months through 8 years, received two doses of influenza vaccine last season, or at least one dose two or more years ago; 9 years and older, any vaccination history | ||
| 6 months through 35 months | 0.25 mL/Intramuscular | 1 dose |
| 36 months and older | 0.5 mL/Intramuscular | 1 dose |
| 65 years and older, any influenza vaccination history | Dose/Route | Schedule |
| 0.5 mL/Intramuscular | 1 dose |
| 18 through 64 years of age, any influenza vaccination history | Dose/Route | Schedule |
| 0.1 mL/Intradermal | 1 dose |
2.2. Administration
Inspect Fluzone and Fluzone High-Dose syringes and vials and Fluzone Intradermal microinjection system visually for particulate matter and/or discoloration prior to administration. If either of these conditions exist, the vaccine should not be administered.
Fluzone
Shake the syringe or single-dose vial before administering the vaccine and shake the multi-dose vial each time before withdrawing a dose of vaccine.
The preferred sites for intramuscular injection are the anterolateral aspect of the thigh in infants 6 months through 11 months of age, or the deltoid muscle in persons ≥12 months of age.
Fluzone High-Dose
Shake the syringe before administering the vaccine.
The preferred site for intramuscular injection is the deltoid muscle.
Fluzone Intradermal
Gently shake the microinjection system before administering the vaccine.
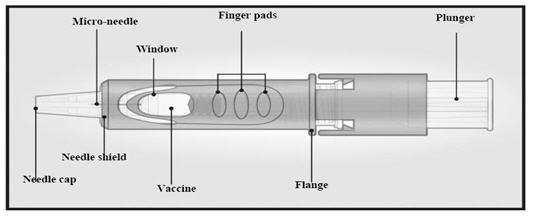
1. Remove Needle Cap
Remove the needle cap from the microinjection system.
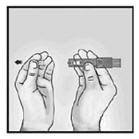
2. Hold Microinjection System Between Thumb and Middle Finger
Hold the system by placing the thumb and middle finger only on the finger pads, the index finger remains free. Do not place fingers on the windows.
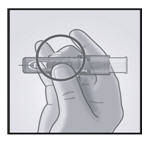
3. Insert Needle Rapidly and Perpendicular to the Skin
Insert the needle perpendicular to the skin, in the region of the deltoid, in a short, quick movement.
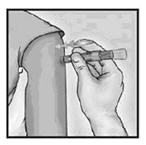
4. Inject Using the Index Finger
Once the needle has been inserted, maintain light pressure on the surface of the skin and inject using the index finger to push on the plunger. Do not aspirate.
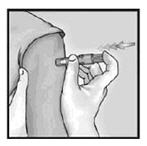
5. Remove Needle from Skin and Activate Needle Shield by Pushing Firmly on Plunger
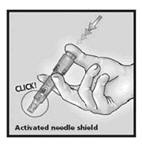
Remove the needle from the skin. Direct the needle away from you and others. With the same hand, push very firmly with the thumb on the plunger to activate the needle shield. You will hear a click when the shield extends to cover the needle.
3. DOSAGE FORMS AND STRENGTHS
Fluzone, Fluzone-High Dose, and Fluzone Intradermal are suspensions for injection.
Fluzone
Fluzone is supplied in 4 presentations:
- 1)
- Prefilled syringe (pink syringe plunger rod), 0.25 mL, for persons 6 months through 35 months of age.
- 2)
- Prefilled syringe (clear syringe plunger rod), 0.5 mL, for persons 36 months of age and older.
- 3)
- Single-dose vial, 0.5 mL, for persons 36 months of age and older.
- 4)
- Multi-dose vial, 5 mL, for persons 6 months of age and older.
4. CONTRAINDICATIONS
A severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)], including egg protein, or to a previous dose of any influenza vaccine is a contraindication to administration of Fluzone, Fluzone High-Dose or Fluzone Intradermal.
5. WARNINGS AND PRECAUTIONS
5.1. Guillain-Barré Syndrome
The 1976 swine influenza vaccine was associated with an elevated risk of Guillain-Barré syndrome (GBS). Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated (1). If GBS has occurred within 6 weeks of previous influenza vaccination, the decision to give Fluzone, Fluzone High-Dose or Fluzone Intradermal should be based on careful consideration of the potential benefits and risks.
5.2. Latex
The prefilled syringe tip caps of Fluzone and Fluzone High-Dose may contain natural rubber latex which may cause allergic reactions in latex sensitive individuals.
5.3. Preventing and Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trial of another vaccine, and may not reflect the rates observed in practice.
Fluzone – Children 6 Months through 8 Years of Age
In a multi-center study conducted in the US, children 6 months through 35 months of age received two 0.25 mL doses of Fluzone, and children 3 years through 8 years of age received two 0.5 mL doses of Fluzone, irrespective of previous influenza vaccination history. The two doses (year 2006-2007 formulation) were administered 26 to 30 days apart. The safety analysis set included 97 children 6 months through 35 months of age and 163 children 3 years through 8 years of age. Table 4 and Table 5 summarize solicited injection site reactions and systemic adverse events reported within 7 days post-vaccination via diary cards.
| Dose 1 (N*=90-92) Percentage | Dose 2 (N*=86-87) Percentage |
|||||
|---|---|---|---|---|---|---|
| Any | Moderate† | Severe‡ | Any | Moderate† | Severe‡ | |
|
||||||
| Injection-Site Tenderness | 47.3 | 8.8 | 0.0 | 56.3 | 3.4 | 1.1 |
| Injection-Site Erythema | 29.3 | 0.0 | 0.0 | 32.2 | 1.1 | 0.0 |
| Injection-Site Swelling | 16.7 | 0.0 | 0.0 | 14.9 | 0.0 | 0.0 |
| Injection-Site Induration | 14.4 | 0.0 | 0.0 | 16.1 | 0.0 | 0.0 |
| Injection-Site Ecchymosis | 14.4 | 1.1 | 0.0 | 14.9 | 2.3 | 0.0 |
| Fever§ | 11.0 | 4.4 | 0.0 | 10.3 | 3.4 | 1.1 |
| Vomiting | 6.6 | 1.1 | 0.0 | 8.1 | 5.8 | 0.0 |
| Crying Abnormal | 31.9 | 11.0 | 0.0 | 18.6 | 7.0 | 2.3 |
| Drowsiness | 26.4 | 1.1 | 0.0 | 26.7 | 4.7 | 0.0 |
| Appetite Lost | 23.1 | 8.8 | 0.0 | 19.8 | 5.8 | 1.2 |
| Irritability | 42.9 | 19.8 | 1.1 | 34.9 | 17.4 | 4.7 |
| Dose 1 (N*=150-151) Percentage | Dose 2 (N*=144-145) Percentage |
|||||
|---|---|---|---|---|---|---|
| Any | Moderate† | Severe‡ | Any | Moderate† | Severe‡ | |
| "-" indicates information was not collected | ||||||
|
||||||
| Injection-Site Pain | 59.3 | 8.0 | 0.0 | 62.1 | 9.7 | 0.7 |
| Injection-Site Erythema | 27.8 | 3.3 | 0.7 | 27.6 | 2.1 | 0.7 |
| Injection-Site Swelling | 19.9 | 5.3 | 0.0 | 14.5 | 2.8 | 0.0 |
| Injection-Site Induration | 16.6 | 2.0 | 0.0 | 11.7 | 1.4 | 0.0 |
| Injection-Site Ecchymosis | 12.6 | 0.7 | 0.7 | 15.2 | 0.7 | 0.0 |
| Injection-Site Pruritus | 7.3 | - | - | 13.2 | - | - |
| Fever§ | 11.9 | 2.6 | 2.0 | 9.7 | 1.4 | 1.4 |
| Headache | 16.7 | 2.0 | 0.7 | 11.8 | 1.4 | 1.4 |
| Malaise | 20.0 | 2.7 | 1.3 | 14.6 | 4.2 | 0.7 |
| Myalgia | 28.0 | 5.3 | 0.0 | 17.4 | 4.2 | 0.0 |
During the period from the first vaccination through 6 months following the second vaccination, there were no serious adverse events considered to be caused by vaccination and no deaths reported in this study.
Fluzone and Fluzone Intradermal – Adults
Adults 18 through 64 years of age were randomized to receive Fluzone Intradermal or Fluzone (year 2008-2009 formulation) in a multi-center trial conducted in the US. The trial was open-label for administration route. The safety analysis set included 2855 Fluzone Intradermal recipients and 1421 Fluzone recipients. Table 6 summarizes solicited injection-site reactions and systemic adverse events reported within 7 days post-vaccination via diary cards. With the exception of pain, solicited injection-site reactions were more frequent after vaccination with Fluzone Intradermal compared to Fluzone. Nine percent of Fluzone recipients and 49% of Fluzone Intradermal recipients had an injection-site reaction present beyond Day 3 post-vaccination. Approximately 20% of subjects in both groups had a solicited systemic adverse event present beyond Day 3 post-vaccination.
| Fluzone Intradermal (N*=2798-2802) Percentage | Fluzone (N*=1392-1394) Percentage |
|||||
|---|---|---|---|---|---|---|
| Any | Grade 2† | Grade 3‡ | Any | Grade 2† | Grade 3‡ | |
|
||||||
| Injection-Site Erythema | 76.4 | 28.8 | 13.0 | 13.2 | 2.1 | 0.9 |
| Injection-Site Induration | 58.4 | 13.0 | 3.4 | 10.0 | 2.3 | 0.5 |
| Injection-Site Swelling | 56.8 | 13.4 | 5.4 | 8.4 | 2.1 | 0.9 |
| Injection-Site Pain | 51.0 | 4.4 | 0.6 | 53.7 | 5.8 | 0.8 |
| Injection-Site Pruritus | 46.9 | 4.1 | 1.1 | 9.3 | 0.4 | 0.0 |
| Injection-Site Ecchymosis | 9.3 | 1.4 | 0.4 | 6.2 | 1.1 | 0.4 |
| Headache | 31.2 | 6.4 | 1.5 | 30.3 | 6.5 | 1.6 |
| Myalgia | 26.5 | 4.6 | 1.5 | 30.8 | 5.5 | 1.4 |
| Malaise | 23.3 | 5.5 | 2.2 | 22.2 | 5.5 | 1.8 |
| Shivering | 7.3 | 1.5 | 0.7 | 6.2 | 1.1 | 0.6 |
| Fever§ | 3.9 | 0.6 | 0.1 | 2.6 | 0.4 | 0.2 |
Within 28 days post-vaccination, a serious adverse event was reported by 10 (0.4%) Fluzone Intradermal recipients and 5 (0.4%) Fluzone recipients. Within 6 months post-vaccination, a serious adverse event was reported by 47 (1.6%) Fluzone Intradermal recipients and 20 (1.4%) Fluzone recipients. No deaths were reported during the 6 months post-vaccination. Throughout the study, one reported serious adverse event was considered to be caused by vaccination: a pruritic rash on the extremities and torso that began 48 hours after receipt of Fluzone Intradermal and resulted in hospitalization and treatment with an antihistamine and steroids.
Fluzone and Fluzone High-Dose – Geriatric Adults
Adults 65 years of age and older were randomized to receive either Fluzone High-Dose or Fluzone (year 2006-2007 formulation) in a multi-center, double-blind trial conducted in the US. The safety analysis set included 2573 Fluzone High-Dose recipients and 1260 Fluzone recipients.
Table 7 summarizes solicited injection-site reactions and systemic adverse events reported within 7 days post-vaccination via diary cards. Onset was usually within the first 3 days after vaccination and a majority of the reactions resolved within 3 days. Solicited injection-site reactions and systemic adverse events were more frequent after vaccination with Fluzone High-Dose compared to Fluzone.
| Fluzone High-Dose (N*=2569-2572) Percentage | Fluzone (N*=1258-1260) Percentage |
|||||
|---|---|---|---|---|---|---|
| Any | Moderate† | Severe‡ | Any | Moderate† | Severe‡ | |
|
||||||
| Injection-Site Pain | 35.6 | 3.7 | 0.3 | 24.3 | 1.7 | 0.2 |
| Injection-Site Erythema | 14.9 | 1.9 | 1.8 | 10.8 | 0.8 | 0.6 |
| Injection-Site Swelling | 8.9 | 1.6 | 1.5 | 5.8 | 1.3 | 0.6 |
| Myalgia | 21.4 | 4.2 | 1.6 | 18.3 | 3.2 | 0.2 |
| Malaise | 18.0 | 4.7 | 1.6 | 14.0 | 3.7 | 0.6 |
| Headache | 16.8 | 3.1 | 1.1 | 14.4 | 2.5 | 0.3 |
| Fever§ | 3.6 | 1.1 | 0.0 | 2.3 | 0.2 | 0.1 |
Within 6 months post-vaccination, 156 (6.1%) Fluzone High-Dose recipients and 93 (7.4%) Fluzone recipients experienced a serious adverse event. No deaths were reported within 28 days post-vaccination. A total of 23 deaths were reported during the period Day 29-180 post-vaccination: 16 (0.6%) among Fluzone High-Dose recipients and 7 (0.6%) among Fluzone recipients. The majority of these participants had a medical history of cardiac, hepatic, neoplastic, renal, and/or respiratory diseases.
6.2. Post-Marketing Experience
The following events have been spontaneously reported during the post-approval use of Fluzone or Fluzone High-Dose. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strengh of evidence for a causal relationship to Fluzone or Fluzone High-Dose.
Events Reported During Post-Approval Use of Fluzone.
- Blood and Lymphatic System Disorders: Thrombocytopenia, lymphadenopathy
- Immune System Disorders: Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema)
- Nervous System Disorders: Guillain-Barré syndrome (GBS), convulsions, febrile convulsions, myelitis (including encephalomyelitis and transverse myelitis), facial palsy (Bell's palsy), optic neuritis/neuropathy, brachial neuritis, syncope (shortly after vaccination), dizziness, paresthesia
- Vascular Disorders: Vasculitis, vasodilatation/flushing
- Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, pharyngitis, rhinitis
- Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
- General Disorders and Administration Site Conditions: Pruritus, asthenia/fatigue, pain in extremities, chest pain
Events Reported During Post-Approval Use of Fluzone High-Dose.
- Gastrointestinal Disorders: Nausea, vomiting, diarrhea
7. DRUG INTERACTIONS
Data evaluating the concomitant administration of Fluzone, Fluzone High-Dose, or Fluzone Intradermal with other vaccines are not available.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Fluzone and Fluzone High-Dose
Pregnancy Category C: Animal reproduction studies have not been conducted with Fluzone or Fluzone High-Dose. It is also not known whether Fluzone or Fluzone High-Dose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Fluzone or Fluzone High-Dose should be given to a pregnant woman only if clearly needed.
Fluzone Intradermal
Pregnancy Category B: A developmental and reproductive toxicity study has been performed in female rabbits at a dose approximately 20 times the human dose (on a mg/kg basis) and has revealed no evidence of impaired female fertility or harm to the fetus due to Fluzone Intradermal. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Fluzone Intradermal should be used during pregnancy only if clearly needed.
Healthcare providers are encouraged to register women who receive Fluzone Intradermal during pregnancy in Sanofi Pasteur Inc.'s vaccination pregnancy registry by calling 1-800-822-2463.
8.3. Nursing Mothers
It is not known whether Fluzone or Fluzone Intradermal is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Fluzone or Fluzone Intradermal is administered to a nursing woman.
8.4. Pediatric Use
Fluzone
Safety and effectiveness of Fluzone in children below the age of 6 months have not been established. Safety and immunogenicity of Fluzone was evaluated in children 6 months through 8 years of age. [See Adverse Reactions (6.1) and Clinical Studies (14.1).]
Fluzone High-Dose
Safety and effectiveness of Fluzone High-Dose in persons <65 years of age have not been established.
Fluzone Intradermal
Safety and effectiveness of Fluzone Intradermal in persons <18 years of age have not been established. In a clinical trial, 97 infants and toddlers 6 months through 35 months of age and 160 children 3 years through 8 years of age were enrolled to receive two injections of Fluzone Intradermal. Infants and children in a control group received two injections of Fluzone. Fluzone Intradermal was associated with increased local reactogenicity relative to Fluzone. The size of the study was not adequate to reliably evaluate serious adverse events or the immune response elicited by Fluzone Intradermal relative to Fluzone.
8.5. Geriatric Use
Fluzone
In two observational studies of Fluzone in 118 adults 19 through 59 years of age and 123 adults 61 through 86 years of age, the immune responses were lower in the older adults.
[See Clinical Studies (14.3).]
Fluzone High-Dose
Safety and immunogenicity of Fluzone High-Dose have been evaluated in adults 65 years of age and older. [See Adverse Reactions (6.1) and Clinical Studies (14.3).]
11. DESCRIPTION
Fluzone (Influenza Virus Vaccine) for intramuscular injection, Fluzone High-Dose (Influenza Virus Vaccine) for intramuscular injection, and Fluzone Intradermal (Influenza Virus Vaccine) for intradermal injection are inactivated influenza virus vaccines, prepared from influenza viruses propagated in embryonated chicken eggs. The virus-containing allantoic fluid is harvested and inactivated with formaldehyde. Influenza virus is concentrated and purified in a linear sucrose density gradient solution using a continuous flow centrifuge. The virus is then chemically disrupted using a non-ionic surfactant, Octylphenol Ethoxylate (Triton® X-100), producing a "split virus". The split virus is further purified and then suspended in sodium phosphate-buffered isotonic sodium chloride solution. The Fluzone High-Dose and Fluzone Intradermal processes use an additional concentration factor after the ultrafiltration step in order to obtain a higher hemagglutinin (HA) antigen concentration.
Fluzone, Fluzone High-Dose, and Fluzone Intradermal, suspensions for injection, are clear and slightly opalescent in color.
Antibiotics are not used in the manufacture of Fluzone, Fluzone High-Dose or Fluzone Intradermal.
The prefilled syringe tip caps of Fluzone and Fluzone High-Dose may contain natural rubber latex. Vial presentations of Fluzone and the Fluzone Intradermal microinjection system do not contain latex.
Fluzone, Fluzone High-Dose, and Fluzone Intradermal are standardized according to United States Public Health Service requirements and are formulated to contain HA of each of the following three influenza strains recommended for the 2011-2012 influenza season: A/California/07/2009 X-179A (H1N1), A/Victoria/210/2009 X-187 (an A/Perth/16/2009-like strain) (H3N2) and B/Brisbane/60/2008. The amounts of HA and other ingredients per dose of vaccine are listed in Table 8.
| Quantity (per dose) |
||||
|---|---|---|---|---|
| Ingredient | Fluzone 0.25 mL Dose | Fluzone 0.5 mL Dose | Fluzone High-Dose 0.5 mL Dose | Fluzone Intradermal 0.1 mL Dose |
| N/A not applicable | ||||
| Active Substance: Split influenza virus, inactivated strains*: | 22.5 mcg HA total | 45 mcg HA total | 180 mcg HA total | 27 mcg HA total |
| A (H1N1) | 7.5 mcg HA | 15 mcg HA | 60 mcg HA | 9 mcg HA |
| A (H3N2) | 7.5 mcg HA | 15 mcg HA | 60 mcg HA | 9 mcg HA |
| B | 7.5 mcg HA | 15 mcg HA | 60 mcg HA | 9 mcg HA |
| Other: | ||||
| Sodium phosphate-buffered isotonic sodium chloride solution | QS† to appropriate volume | QS† to appropriate volume | QS† to appropriate volume | QS† to appropriate volume |
| Formaldehyde | ≤50 mcg | ≤100 mcg | ≤100 mcg | ≤20 mcg |
| Octylphenol Ethoxylate | ≤75 mcg | ≤150 mcg | ≤250 mcg | ≤50 mcg |
| Gelatin | 0.05% | 0.05% | None | None |
| Preservative | ||||
| Single-Dose Presentations | None | None | None | None |
| Multi-Dose Presentation (Thimerosal) | N/A | 25 mcg mercury | N/A | N/A |
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance of influenza identifies yearly antigenic variants. For example, since 1977, antigenic variants of influenza A (H1N1 and H3N2) viruses and influenza B viruses have been in global circulation. Specific levels of hemagglutination inhibition (HI) antibody titer post-vaccination with inactivated influenza virus vaccines have not been correlated with protection from influenza virus infection. In some human studies, antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of subjects. (2) (3)
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more new strains in each year's influenza vaccine. Therefore, influenza vaccines are standardized to contain the hemagglutinins of influenza virus strains (ie, typically two type A and one type B), representing the influenza viruses likely to be circulating in the US in the upcoming winter.
Annual vaccination with the current vaccine is recommended because immunity during the year after vaccination declines, and because circulating strains of influenza virus change from year to year. (4)
14. CLINICAL STUDIES
14.1. Immunogenicity of Fluzone in Children 6 Months through 8 Years of Age
In a multi-center study conducted in the US, 68 children 6 months through 35 months of age given two 0.25 mL doses of Fluzone and 120 children 3 years through 8 years of age given two 0.5 mL doses of Fluzone were included in the per-protocol analysis set. The two doses (year 2006-2007 formulation) were administered 26 to 30 days apart. Females accounted for 42.6% of the participants in the 6 months through 35 months age group and 53.3% of the participants in the 3 years through 8 years age group. Most participants in the 6 months through 35 months and 3 years through 8 years age groups, respectively, were Caucasian (70.6% and 79.2%), followed by Hispanic (19.1% and 13.3%), and Black (7.4% and 4.2%).
The percentage of participants who received influenza vaccination during the previous influenza season was 54.4% for the 6 months through 35 months age group and 27.5% for the 3 years through 8 years age group. Table 9 shows seroconversion rates and the percentage of participants with an HI titer ≥1:40 one month following the second dose of Fluzone.
| Antigen | Age Group | Titer ≥1:40 % (95% CI) | Seroconversion†
% (95% CI) |
|---|---|---|---|
|
|||
| A (H1N1) | 6 through 35 months (N=68) | 92.6 (83.7; 97.6) | 88.2 (78.1; 94.8) |
| 3 through 8 years (N=120) | 99.2 (95.4; 100.0) | 78.3 (69.9; 85.3) | |
| A (H3N2) | 6 through 35 months (N=68) | 100.0 (94.7; 100.0) | 91.2 (81.8; 96.7) |
| 3 through 8 years (N=120) | 100.0 (97.0; 100.0) | 61.7 (52.4; 70.4) | |
| B | 6 through 35 months (N=68) | 20.6 (11.7; 32.1) | 20.6 (11.7; 32.1) |
| 3 through 8 years (N=120) | 58.3 (49.0; 67.3) | 53.3 (44.0; 62.5) | |
14.2. Immunogenicity of Fluzone and Fluzone Intradermal in Adults
Adults 18 through 64 years of age were randomized to receive Fluzone Intradermal or Fluzone (year 2008-2009 formulation) in a multi-center trial conducted in the US. The trial was open-label for administration route. For immunogenicity analyses, there were 2581 participants who received Fluzone Intradermal and 1287 participants who received Fluzone in the per protocol analysis set. There were fewer males than females (36.1% and 35.8% males in the Fluzone Intradermal and Fluzone groups, respectively). In the Fluzone Intradermal group, the mean age was 42.7 years (ranged from 18.1 through 65.0 years), and in the Fluzone group, the mean age was 42.6 years (ranged from 18.2 through 65.0 years). Most participants in the Fluzone Intradermal and Fluzone groups, respectively, were Caucasian (79.6% and 80.0%), followed by Hispanic (10.2% and 11.0%), and Black (7.7% and 6.3%). HI antibody geometric mean titers (GMTs) following Fluzone Intradermal were non-inferior to those following Fluzone for all three strains. (See Table 10) Seroconversion rates following Fluzone Intradermal were non-inferior to those following Fluzone for strains A (H1N1) and A (H3N2), but not for strain B. (See Table 11) At 28 days following vaccination with either Fluzone or Fluzone Intradermal, the percentages of subjects with a serum HI antibody titer of at least 1:40 ranged from 87% to 92%, depending on the influenza strain.
| GMT | GMT Ratio (95% CI) | Non-inferior* | ||
|---|---|---|---|---|
| Influenza Strain | Fluzone Intradermal N=2575-2579 | Fluzone N=1283-1285 | Fluzone GMT divided by Fluzone Intradermal GMT | |
|
||||
| A (H1N1) | 193.2 | 178.3 | 0.92 (0.85; 1.01) | Yes |
| A (H3N2) | 246.7 | 230.7 | 0.94 (0.85; 1.03) | Yes |
| B | 102.5 | 126.9 | 1.24 (1.15; 1.33) | Yes |
| Seroconversion* % | Difference (95% CI) | Non-inferior† | ||
|---|---|---|---|---|
| Influenza Strain | Fluzone Intradermal N=2573-2578 | Fluzone N=1283-1285 | Fluzone minus Fluzone Intradermal | |
|
||||
| A (H1N1) | 61.2 | 60.5 | -0.69 (-3.97; 2.56) | Yes |
| A (H3N2) | 75.3 | 74.8 | -0.55 (-3.49; 2.31) | Yes |
| B | 46.2 | 54.2 | 7.99 (4.64; 11.31) | No |
14.3. Immunogenicity of Fluzone in Adults and Geriatric Adults
In two observational studies, the immunogenicity of a single dose of Fluzone (year 1999-2000 and year 2000-2001 formulation, respectively) was evaluated in adults 19 through 59 years of age (median age: 38 years) and adults 61 through 86 years of age (median age: 72 years). The percentages of subjects with post-vaccination HI titers ≥1:40 and a four-fold increase in titer from pre-vaccination to post-vaccination were analyzed. (See Table 12)
| Antigen | Influenza Season | Age Group | Titer ≥1:40 Percent | 4-Fold Increase Percent |
|---|---|---|---|---|
| N = Number of participants. | ||||
| A (H1N1) | 1999-2000 | 19-59 Years (N = 60) | 49 | 34 |
| 61-86 Years (N = 61) | 38 | 27 | ||
| 2000-2001 | 19-59 Years (N = 58) | 54 | 52 | |
| 61-86 Years (N = 62) | 23 | 39 | ||
| A (H3N2) | 1999-2000 | 19-59 Years (N = 60) | 72 | 46 |
| 61-86 Years (N = 61) | 70 | 42 | ||
| 2000-2001 | 19-59 Years (N = 58) | 79 | 45 | |
| 61-86 Years (N = 62) | 68 | 44 | ||
| B | 1999-2000 | 19-59 Years (N = 60) | 38 | 30 |
| 61-86 Years (N = 61) | 10 | 10 | ||
| 2000-2001 | 19-59 Years (N = 58) | 38 | 29 | |
| 61-86 Years (N = 62) | 11 | 5 | ||
14.4. Immunogencity of Fluzone and Fluzone High-Dose in Geriatric Adults
Adults 65 years of age and older were randomized to receive either Fluzone High-Dose or Fluzone (year 2006-2007 formulation) in a multi-center, double-blind trial conducted in the US. For immunogenicity analyses, 2,576 participants were randomized to Fluzone High-Dose and 1,275 participants were randomized to Fluzone. Females accounted for 51.3% of participants in the Fluzone High-Dose group and 54.7% of participants in the Fluzone group. In both groups, the mean age was 72.9 years (ranged from 65 through 97 years in the Fluzone High-Dose group and 65 through 94 years in the Fluzone group); 35% of participants in the Fluzone High-Dose group and 36% of participants in the Fluzone group were 75 years of age or older. Most participants in the Fluzone High-Dose and Fluzone groups, respectively, were Caucasian (91.7% and 92.9%), followed by Hispanic (4.8% and 3.7%), and Black (2.7% and 2.7%).
The primary endpoints of the study were HI GMTs and seroconversion rates 28 days after vaccination. Pre-specified statistical superiority criteria required that the lower limit (LL) of the 2-sided 95% CI of the GMT ratio [Fluzone High-Dose/Fluzone] be greater than 1.50 for at least two of the strains, and if one strain failed, non-inferiority of that strain must be demonstrated (LL>0.67), and that the lower limit of the 2-sided 95% CI of the seroconversion rate difference [Fluzone High-Dose minus Fluzone] be greater than 10% for at least two of the strains, and if one strain failed, non-inferiority of that strain must be demonstrated (LL>-10%). As shown in Table 13, statistically superior HI GMTs and seroconversion rates after vaccination with Fluzone High-Dose compared to Fluzone were demonstrated for influenza A subtypes, A (H1N1) and A (H3N2), but not for influenza type B. For strain B, non-inferiority of Fluzone High-Dose compared to Fluzone was demonstrated for both the HI GMTs and seroconversion rates. There are no data demonstrating clinically relevant prevention of culture-confirmed influenza or its complications after vaccination with Fluzone High-Dose compared to Fluzone in individuals 65 years of age and older.
| GMT | GMT Ratio | Seroconversion %* | Difference | Met Both Pre-defined Superiority Criteria† | |||
|---|---|---|---|---|---|---|---|
| Influenza Strain | Fluzone High-Dose N‡=2576 | Fluzone N‡=1275 | Fluzone High-Dose over Fluzone (95% CI) | Fluzone High-Dose N‡=2576 | Fluzone N‡=1275 | Fluzone High-Dose minus Fluzone (95% CI) | |
|
|||||||
| A (H1N1) | 115.8 | 67.3 | 1.7 (1.6; 1.8) | 48.6 | 23.1 | 25.4 (22.4; 28.5) | Yes |
| A (H3N2) | 608.9 | 332.5 | 1.8 (1.7; 2.0) | 69.1 | 50.7 | 18.4 (15.1; 21.7) | Yes |
| B | 69.1 | 52.3 | 1.3 (1.2; 1.4) | 41.8 | 29.9 | 11.8 (8.6; 15.0) | No |
15. REFERENCES
- 1
- Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barre´ syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998;339(25):1797-802.
- 2
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133-138.
- 3
- Hobson D, Curry RL, Beare AS, Ward-Gardner Al. The role of serum haemagglutintion-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972;767-777.
- 4
- Centers for Disease Control and Prevention. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(RR-8):1-68.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
The prefilled syringe tip caps of Fluzone and Fluzone High-Dose may contain natural rubber latex. Vial presentations of Fluzone and the Fluzone Intradermal microinjection system do not contain latex.
Fluzone
Single-dose, prefilled syringe, without needle, 0.25 mL, package of 10 (may contain latex) – NDC 49281-111-25.
Single-dose, prefilled syringe, without needle, 0.5 mL, package of 10 (may contain latex) – NDC 49281-011-50.
Single-dose vial, 0.5 mL, package of 10 (does not contain latex) – NDC 49281-011-10.
Multi-dose vial, 5 mL, package of one (does not contain latex). The vial contains ten 0.5 mL doses – NDC 49281-388-15.
16.2. Storage and Handling
Store all Fluzone, Fluzone High-Dose, and Fluzone Intradermal presentations refrigerated at 2° to 8°C (35° to 46°F). DO NOT FREEZE. Discard if vaccine has been frozen.
Between uses, return the multi-dose vial to the recommended storage conditions at 2° to 8°C (35° to 46°F).
Do not use after the expiration date shown on the label.
17. PATIENT COUNSELING INFORMATION
Inform the patient or guardian that Fluzone, Fluzone High-Dose, and Fluzone Intradermal contain killed viruses and cannot cause influenza. Fluzone, Fluzone High-Dose, and Fluzone Intradermal stimulate the immune system to produce antibodies that help protect against influenza, but do not prevent other respiratory infections. Annual influenza vaccination is recommended. Instruct vaccine recipients and guardians to report adverse reactions to their healthcare provider and/or to VAERS. Inform the patient about the Sanofi Pasteur Inc. pregnancy registry, for Fluzone Intradermal as appropriate.
Fluzone is a registered trademark of Sanofi Pasteur Inc.
Product information as of May 2011.
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
6109/6044/6045/6046
Patient Information Sheet
Fluzone®/Fluzone® High-Dose/Fluzone® Intradermal
Influenza Virus Vaccine
Please read this information sheet before getting Fluzone®, Fluzone® High-Dose, or Fluzone® Intradermal vaccine. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What are Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines?
Fluzone is a vaccine that helps protect against influenza illness (flu).
Fluzone vaccine is for people who are 6 months of age and older.
Fluzone High-Dose vaccine is a higher dose of Fluzone for people 65 years of age and older.
Fluzone Intradermal vaccine is for people 18 through 64 years of age.
Vaccination with Fluzone, Fluzone High-Dose, or Fluzone Intradermal vaccine may not protect all people who receive the vaccine.
Who should not get Fluzone, Fluzone High-Dose, or Fluzone Intradermal vaccine?
You should not get Fluzone, Fluzone High-Dose, or Fluzone Intradermal vaccine if you:
- ever had a severe allergic reaction to eggs or egg products..
- ever had a severe allergic reaction after getting any flu shot.
- are younger than 6 months of age.
Tell your healthcare provider if you or your child have or have had:
- Guillain-Barré syndrome (severe muscle weakness) after getting a flu shot.
- problems with your immune system as the immune response may be diminished.
- an allergy to rubber latex.
How are Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines given?
Fluzone or Fluzone High-Dose vaccine is a shot given into the muscle of the arm.
For infants, Fluzone vaccine is a shot given into the muscle of the thigh.
Fluzone Intradermal vaccine is a shot given into the skin of the arm.
What are the possible side effects of Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines?
The most common side effects of Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines are:
- pain, redness, swelling, hardness, and itching where you got the shot
- muscular pain
- tiredness
- headache
- fever
These are not all of the possible side effects of Fluzone, Fluzone High-Dose, or Fluzone Intradermal vaccine. You can ask your healthcare provider for a list of other side effects that is available to healthcare professionals.
Call your healthcare provider for advice about any side effects that concern you. You may report side effects to VAERS at 1-800-822-7967 or http://vaers.hhs.gov. A pregnancy registry is available for Fluzone Intradermal by contacting Sanofi Pasteur Inc. at 1-800-822-2463.
What are the ingredients in Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines?
Fluzone, Fluzone High-Dose, and Fluzone Intradermal vaccines contain 3 killed flu virus strains.
Inactive ingredients include formaldehyde, octylphenol ethoxylate, and gelatin (no gelatin in Fluzone High-Dose or Fluzone Intradermal).
The preservative thimerosal is only in the 10 dose (multi-dose) vial of Fluzone vaccine.
PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
NDC 49281-388-15
Influenza
Virus
Vaccine
Fluzone®
Flu
5 mL
Rx only
PRINCIPAL DISPLAY PANEL - 1 Vial Carton
NDC 49281-388-15
Influenza
Virus Vaccine
Fluzone®
For 6 months of age and older
2011-2012 Formula
5 mL
Rx only
sanofi pasteur

PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
NDC 49281-011-10
Influenza Virus
Vaccine
Fluzone®
No Preservative
2011-2012 Formula
Mfd by: Sanofi Pasteur Inc.
Flu
1 Dose (0.5 mL)
IM only
Rx only

PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC 49281-011-10
Influenza
Virus Vaccine
Fluzone®
For 36 months of age and older
2011 – 2012 Formula
10 VIALS
0.5 mL
each
Rx only
sanofi pasteur
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
NDC 49281-011-50
0.5 mL Dose
Rx only
Flu
Influenza Virus Vaccine
Fluzone®
IM only
No Preservative
2011-2012 Formula
Mfd by: Sanofi Pasteur Inc.

PRINCIPAL DISPLAY PANEL -10 Syringe Carton
NDC 49281-011-50
Influenza
Virus
Vaccine
Fluzone®
For 36 months of age and older
2011 – 2012 Formula
10 Prefilled
Syringes
0.5 mL each
Rx only
sanofi pasteur

PRINCIPAL DISPLAY PANEL - 0.25 mL Syringe Label
NDC 49281-111-25
0.25 mL Dose
Rx only
Flu
Influenza Virus Vaccine
Fluzone®
IM only
No Preservative: Pediatric Dose
2011-2012 Formula
Mfd by: Sanofi Pasteur Inc.
PRINCIPAL DISPLAY PANEL - 10 Syringe Carton
Pediatric Dose
NDC 49281-111-25
Influenza
Virus
Vaccine
Fluzone®
PEDIATRIC DOSE
FOR 6-35 MONTHS OF AGE
2011 – 2012 Formula
10 Prefilled
Syringes
0.25 mL each
Rx only
sanofi pasteur
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
NDC 49281-389-65
0.5 mL Dose
Rx only
Influenza Virus Vaccine
Fluzone® High-Dose
2011-2012 Formula
IM only
For 65 yrs of age and older
Mfd by: Sanofi Pasteur Inc.

PRINCIPAL DISPLAY PANEL - 10 Syringe Carton
NDC 49281-389-65
65+
Influenza Virus Vaccine
Fluzone® High-Dose
For 65 years of age and older
Rx only
2011-2012 Formula
10 Prefilled Syringes
0.5 mL each
sanofi pasteur

PRINCIPAL DISPLAY PANEL - 0.1 mL Syringe Label
NDC 49281-703-55
0.1 mL Dose
Influenza Virus Vaccine
Fluzone® Intradermal
For 18–64 years of age
Rx only
2011-2012 Formula
For Intradermal Injection Only
Sanofi Pasteur Inc.

PRINCIPAL DISPLAY PANEL - 10 Syringe Carton
NDC 49281-703-55
2011-2012 Formula
Influenza
Virus Vaccine
Fluzone® intradermal
Rx only
10 × 0.1 mL Prefilled
Microinjection Systems
For adults 18 – 64 years of age
sanofi pasteur

| FLUZONE
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103914 | 07/01/2011 | 06/30/2012 |
| FLUZONE
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103914 | 07/01/2011 | 06/30/2012 |
| FLUZONE
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103914 | 07/01/2011 | 06/30/2012 |
| FLUZONE HIGH-DOSE
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103914 | 07/01/2011 | 06/30/2012 |
| FLUZONE INTRADERMAL
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103914 | 07/01/2011 | 06/30/2012 |
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi Pasteur Limited | 208206623 | MANUFACTURE | |
Revised: 07/2011 Sanofi Pasteur Inc.