DOXAZOSIN
-
doxazosin mesylate tablet
Bryant Ranch Prepack
----------
DESCRIPTION
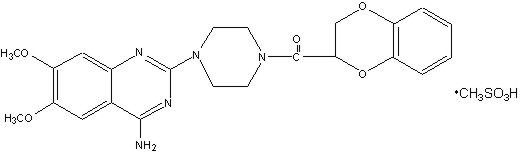
Doxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha subtype of alpha adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. The molecular formula for doxazosin mesylate is C H N O • CH O S and the molecular weight is 547.6. It has the following structure: 123255543
Doxazosin mesylate is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride.
Doxazosin Tablets, USP for oral administration, contain 1 mg, 2 mg, 4 mg or 8 mg of doxazosin as doxazosin mesylate, USP. In addition, each tablet also contains the following inactive ingredients: anhydrous lactose, colloidal silicon, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate and sodium starch glycolate. The 2 mg tablets also contain D&C Red No. 30 Aluminum Lake, the 4 mg tablets contain FD&C Blue No. 2 Aluminum Lake, and the 8 mg tablets contain D&C Red No. 30 Aluminum Lake and FD&C Blue No. 2 Aluminum Lake.
CLINICAL PHARMACOLOGY
Pharmacokinetics
After oral administration of therapeutic doses, peak plasma levels of doxazosin mesylate occur at about 2 to 3 hours. Bioavailability is approximately 65%, reflecting first pass metabolism of doxazosin by the liver. The effect of food on the pharmacokinetics of doxazosin was examined in a crossover study with 12 hypertensive subjects. Reductions of 18% in mean maximum plasma concentration and 12% in the area under the concentration-time curve occurred when doxazosin was administered with food. Neither of these differences was statistically or clinically significant.
Doxazosin is extensively metabolized in the liver, mainly by O-demethylation of the quinazoline nucleus or hydroxylation of the benzodioxan moiety. Although several active metabolites of doxazosin have been identified, the pharmacokinetics of these metabolites have not been characterized. In a study of two subjects administered radiolabelled doxazosin 2 mg orally and 1 mg intravenously on two separate occasions, approximately 63% of the dose was eliminated in the feces and 9% of the dose was found in the urine. On average only 4.8% of the dose was excreted as unchanged drug in the feces and only a trace of the total radioactivity in the urine was attributed to unchanged drug. At the plasma concentrations achieved by therapeutic doses approximately 98% of the circulating drug is bound to plasma proteins.
Plasma elimination of doxazosin is biphasic, with a terminal elimination half-life of about 22 hours. Steady-state studies in hypertensive patients given doxazosin doses of 2 mg to 16 mg once daily showed linear kinetics and dose proportionality. In two studies, following the administration of 2 mg orally once daily, the mean accumulation ratios (steady-state AUC vs. first dose AUC) were 1.2 and 1.7. Enterohepatic recycling is suggested by secondary peaking of plasma doxazosin concentrations.
In a crossover study in 24 normotensive subjects, the pharmacokinetics and safety of doxazosin were shown to be similar with morning and evening dosing regimens. The area under the curve after morning dosing was, however, 11% less than that after evening dosing and the time to peak concentration after evening dosing occurred significantly later than that after morning dosing (5.6 hr vs. 3.5 hr).
The pharmacokinetics of doxazosin in young (< 65 years) and elderly (≥ 65 years) subjects were similar for plasma half-life values and oral clearance. Pharmacokinetic studies in elderly patients and patients with renal impairment have shown no significant alterations compared to younger patients with normal renal function. Administration of a single 2 mg dose to patients with cirrhosis (Child-Pugh Class A) showed a 40% increase in exposure to doxazosin. There are only limited data on the effects of drugs known to influence the hepatic metabolism of doxazosin [e.g., cimetidine (see )]. As with any drug wholly metabolized by the liver, use of doxazosin in patients with altered liver function should be undertaken with caution. PRECAUTIONS: Drug Interactions
In two placebo-controlled studies, of normotensive and hypertensive BPH patients, in which doxazosin was administered in the morning and the titration interval was two weeks and one week, respectively, trough plasma concentrations of doxazosin were similar in the two populations. Linear kinetics and dose proportionality were observed.
CONTRAINDICATIONS
Doxazosin tablets are contraindicated in patients with a known sensitivity to quinazolines (e.g., prazosin, terazosin), doxazosin, or any of the inert ingredients.
PRECAUTIONS
Information for Patients
(See ) Patient Leaflet
Patients should be made aware of the possibility of syncopal and orthostatic symptoms, especially at the initiation of therapy, and urged to avoid driving or hazardous tasks for 24 hours after the first dose, after a dosage increase, and after interruption of therapy when treatment is resumed. They should be cautioned to avoid situations where injury could result should syncope occur during initiation of doxazosin therapy. They should also be advised of the need to sit or lie down when symptoms of lowered blood pressure occur, although these symptoms are not always orthostatic, and to be careful when rising from a sitting or lying position. If dizziness, lightheadedness, or palpitations are bothersome they should be reported to the physician, so that dose adjustment can be considered. Patients should also be told that drowsiness or somnolence can occur with doxazosin or any selective alpha adrenoceptor antagonist, requiring caution in people who must drive or operate heavy machinery. 1
Patients should be advised about the possibility of priapism as a result of treatment with alpha antagonists. Patients should know that this adverse event is very rare. If they experience priapism, it should be brought to immediate medical attention for if not treated promptly it can lead to permanent erectile dysfunction (impotence). 1
Drug/Laboratory Test Interactions
Doxazosin does not affect the plasma concentration of prostate specific antigen in patients treated for up to 3 years. Both doxazosin, an alpha inhibitor, and finasteride, a 5-alpha reductase inhibitor, are highly protein bound and hepatically metabolized. There is no definitive controlled clinical experience on the concomitant use of alpha inhibitors and 5-alpha reductase inhibitors at this time. 11
Drug Interactions
Most (98%) of plasma doxazosin is protein bound. data in human plasma indicate that doxazosin has no effect on protein binding of digoxin, warfarin, phenytoin or indomethacin. There is no information on the effect of other highly plasma protein bound drugs on doxazosin binding. Doxazosin has been administered without any evidence of an adverse drug interaction to patients receiving thiazide diuretics, beta-blocking agents, and nonsteroidal anti-inflammatory drugs. In a placebo-controlled trial in normal volunteers, the administration of a single 1 mg dose of doxazosin on day 1 of a 4-day regimen of oral cimetidine (400 mg twice daily) resulted in a 10% increase in mean AUC of doxazosin (p = 0.006), and a slight but not statistically significant increase in mean C and mean half-life of doxazosin. The clinical significance of this increase in doxazosin AUC is unknown. In vitromax
In clinical trials, doxazosin tablets have been administered to patients on a variety of concomitant medications; while no formal interaction studies have been conducted, no interactions were observed. Doxazosin tablets have been used with the following drugs or drug classes: 1) analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine and codeine combinations, ibuprofen, indomethacin); 2) antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole, amoxicillin); 3) antihistamines (e.g., chlorpheniramine); 4) cardiovascular agents (e.g., atenolol, hydrochlorothiazide, propranolol); 5) corticosteroids; 6) gastrointestinal agents (e.g., antacids); 7) hypoglycemics and endocrine drugs; 8) sedatives and tranquilizers (e.g., diazepam); 9) cold and flu remedies.
Concomitant administration of doxazosin tablets with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension (see ). DOSAGE AND ADMINISTRATION
Carcinogenesis, Mutagenesis, Impairment of Fertility
Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of 40 mg/kg/day in rats and 120 mg/kg/day in mice revealed no evidence of carcinogenic potential. The highest doses evaluated in the rat and mouse studies are associated with AUCs (a measure of systemic exposure) that are 8 times and 4 times, respectively, the human AUC at a dose of 16 mg/day.
Mutagenicity studies revealed no drug- or metabolite-related effects at either chromosomal or subchromosomal levels.
Studies in rats showed reduced fertility in males treated with doxazosin at oral doses of 20 (but not 5 or 10) mg/kg/day, about 4 times the AUC exposures obtained with a 12 mg/day human dose. This effect was reversible within two weeks of drug withdrawal. There have been no reports of any effects of doxazosin on male fertility in humans.
Pregnancy
Teratogenic Effects. Pregnancy Category C
Studies in pregnant rabbits and rats at daily oral doses of up to 41 and 20 mg/kg, respectively (plasma drug concentrations 10 and 4 times human C and AUC exposures with a 12 mg/day therapeutic dose), have revealed no evidence of harm to the fetus. A dosage regimen of 82 mg/kg/day in the rabbit was associated with reduced fetal survival. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, doxazosin should be used during pregnancy only if clearly needed. max
Radioactivity was found to cross the placenta following oral administration of labeled doxazosin to pregnant rats.
Nonteratogenic Effects
In peri-postnatal studies in rats, postnatal development at maternal doses of 40 or 50 mg/kg/day of doxazosin (8 times human AUC exposure with a 12 mg/day therapeutic dose) was delayed as evidenced by slower body weight gain and a slightly later appearance of anatomical features and reflexes.
Nursing Mothers
Studies in lactating rats given a single oral dose of 1 mg/kg of [2- C]-doxazosin indicate that doxazosin accumulates in rat breast milk with a maximum concentration about 20 times greater than the maternal plasma concentration. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxazosin is administered to a nursing mother. 14
OVERDOSAGE
Experience with doxazosin overdosage is limited. Two adolescents who each intentionally ingested 40 mg doxazosin with diclofenac or acetaminophen, were treated with gastric lavage with activated charcoal and made full recoveries. A two-year-old child who accidentally ingested 4 mg doxazosin was treated with gastric lavage and remained normotensive during the five-hour emergency room observation period. A six-month-old child accidentally received a crushed 1 mg tablet of doxazosin and was reported to have been drowsy. A 32-year-old female with chronic renal failure, epilepsy and depression intentionally ingested 60 mg doxazosin (blood level 0.9 mcg/mL; normal values in hypertensives = 0.02 mcg/mL); death was attributed to a grand mal seizure resulting from hypotension. A 39-year-old female who ingested 70 mg doxazosin, alcohol and flurazepam developed hypotension which responded to fluid therapy.
The oral LD of doxazosin is greater than 1000 mg/kg in mice and rats. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of fluid. As doxazosin is highly protein bound, dialysis would not be indicated. 50
DOSAGE AND ADMINISTRATION
The initial dosage of doxazosin tablets in patients with hypertension and/or BPH is 1 mg given once daily in the a.m. or p.m. This starting dose is intended to minimize the frequency of postural hypotension and first dose syncope associated with doxazosin tablets. Postural effects are most likely to occur between 2 and 6 hours after a dose. Therefore blood pressure measurements should be taken during this time period after the first dose and with each increase in dose. If doxazosin tablet administration is discontinued for several days, therapy should be restarted using the initial dosing regimen. DOSAGE MUST BE INDIVIDUALIZED.
Concomitant administration of doxazosin tablets with a PDE-5 inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension; therefore, PDE-5 inhibitor therapy should be initiated at the lowest dose in patients taking doxazosin tablets.
| DOXAZOSIN
doxazosin mesylate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA075509 | 05/27/2011 | |
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bryant Ranch Prepack | 171714327 | REPACK, RELABEL | |
Revised: 04/2011 Bryant Ranch Prepack
