LIALDA
-
mesalamine tablet, delayed release
Shire US Manufacturing Inc.
----------
|
|||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
LIALDA tablets are indicated for the induction of remission in patients with active, mild to moderate ulcerative colitis. Safety and effectiveness of LIALDA beyond 8 weeks has not been established.
2 DOSAGE AND ADMINISTRATION
The recommended dosage for the induction of remission in adult patients with active, mild to moderate ulcerative colitis is two to four 1.2 g tablets to be taken once daily with a meal for a total daily dose of 2.4 g or 4.8 g.
3 DOSAGE FORM AND STRENGTHS
Red-brown ellipsoidal tablet debossed on one side imprinted with S476 containing 1.2 g mesalamine.
4 CONTRAINDICATIONS
LIALDA is contraindicated in patients with hypersensitivity to salicylates (including mesalamine) or to any of the components of LIALDA. [see Description (11)]
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such as LIALDA that contain mesalamine or are converted to mesalamine.
It is recommended that patients have an evaluation of renal function prior to initiation of LIALDA therapy and periodically while on therapy. Exercise caution when using LIALDA in patients with known renal dysfunction or a history of renal disease.
In animal studies, the kidney was the principal organ for toxicity. [see Drug Interactions (7.1) and Nonclinical Toxicology (13.2)]
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, and sometimes fever, headache, and rash. Observe patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with LIALDA.
5.3 Hypersensitivity Reactions
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to LIALDA tablets or to other compounds that contain or are converted to mesalamine.
Mesalamine-induced cardiac hypersensitivity reactions (myocarditis and pericarditis) have been reported with LIALDA and other mesalamine medications. Caution should be taken in prescribing this medicine to patients with conditions predisposing them to the development of myocarditis or pericarditis.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
LIALDA tablets have been evaluated in 655 ulcerative colitis patients in controlled and open-label trials.
In two 8-week placebo-controlled clinical trials involving 535 ulcerative colitis patients, 356 received 2.4 g/day or 4.8 g/day LIALDA tablets and 179 received placebo. More treatment emergent adverse events occurred in the placebo group (119) than in each of the LIALDA treatment groups (109 in 2.4 g/day, 92 in 4.8 g/day). A lower percentage of LIALDA patients discontinued therapy due to adverse events compared to placebo (2.2% vs. 7.3%). The most frequent adverse event leading to discontinuation from LIALDA therapy was exacerbation of ulcerative colitis (0.8%).
The majority of adverse events in the double blind, placebo-controlled trials were mild or moderate in severity. The percentage of patients with severe adverse events was higher in the placebo group (6.1% in placebo; 1.1% in 2.4 g/day; 2.2% in 4.8 g/day). The most common severe adverse events were gastrointestinal disorders which were mainly symptoms associated with ulcerative colitis. Pancreatitis occurred in less than 1% of patients during clinical trials and resulted in discontinuation of therapy with LIALDA in patients experiencing this event.
Overall, the percentage of patients who experienced any adverse event was similar across treatment groups. Treatment related adverse events occurring in LIALDA or placebo groups at a frequency of at least 1% in two Phase 3, 8-week, double blind, placebo-controlled trials are listed in Table 1. The most common treatment related adverse events with LIALDA 2.4 g/day and 4.8 g/day were headache (5.6% and 3.4%, respectively) and flatulence (4% and 2.8%, respectively).
| Event | LIALDA
2.4 g/day (n = 177) | LIALDA
4.8 g/day (n = 179) | Placebo
(n = 179) |
|---|---|---|---|
| Headache | 10 (5.6%) | 6 (3.4%) | 1 (0.6%) |
| Flatulence | 7 (4%) | 5 (2.8%) | 5 (2.8%) |
| Increased alanine aminotransferase | 1 (0.6%) | 2 (1.1%) | 0 |
| Alopecia | 0 | 2 (1.1%) | 0 |
| Pruritus | 1 (0.6%) | 2 (1.1%) | 2 (1.1%) |
|
a: Treatment-emergent related adverse events for which the placebo rate equalled or exceeded the rate for at least one of the LIALDA treatment groups were abdominal pain, dizziness, dyspepsia, and nausea. b: Percentages in this table are based on the treatment-emergent adverse event incidences. |
|||
The following treatment-emergent related adverse events, presented by body system, were reported infrequently (less than 1%) by LIALDA-treated ulcerative colitis patients in the two Phase 3 controlled trials.
Cardiac Disorder: tachycardia
Vascular Disorders: hypertension, hypotension
Skin and Subcutaneous Tissue Disorders: acne, prurigo, rash, urticaria
Gastrointestinal Disorders: abdominal distention, colitis, diarrhea, pancreatitis, rectal polyp, vomiting
Investigations: decreased platelet count, elevated total bilirubin
Musculoskeletal and Connective Tissue Disorders: arthralgia, back pain
Nervous System Disorders: somnolence, tremor
Respiratory, Thoracic and Mediastinal Disorders: pharyngolaryngeal pain
General Disorders and Administrative Site Disorders: asthenia, face edema, fatigue, pyrexia
Ear and Labyrinth Disorders: ear pain
6.2 Postmarketing Experience
In addition to the adverse reactions reported above in clinical trials involving LIALDA, the adverse events listed below have been identified during post-approval use of LIALDA and in controlled clinical trials, open label studies, literature reports, or foreign and domestic marketing experience with other products that contain or are metabolized to mesalamine. Because many of these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: lupus-like syndrome, drug fever
Cardiac Disorders: pericarditis, pericardial effusion, myocarditis
Gastrointestinal: pancreatitis, cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer
Hepatic: jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, Kawasaki-like syndrome including changes in liver enzymes
Hematologic: agranulocytosis, aplastic anemia
Neurological/Psychiatric: peripheral neuropathy, Guillain-Barre syndrome, transverse myelitis
Renal Disorders: interstitial nephritis
Respiratory, Thoracic and Mediastinal Disorders: hypersensitivity pneumonitis (including interstitial pneumonitis, allergic alveolitis, eosinophilic pneumonitis)
Skin: psoriasis, pyoderma gangrenosum, erythema nodosum
Urogenital: reversible oligospermia
7 DRUG INTERACTIONS
No investigations of interaction between LIALDA and other drugs have been performed. However, the following are reports of interactions between mesalamine medications and other drugs.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies with mesalamine have been performed in rats at doses up to 1000 mg/kg/day (1.8 times the maximum recommended human dose based on a body surface area comparison) and rabbits at doses up to 800 mg/kg/day (2.9 times the maximum recommended human dose based on a body surface area comparison) and have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Mesalamine is known to cross the placental barrier.
8.3 Nursing Mothers
Low concentrations of mesalamine and higher concentrations of its N-acetyl metabolite have been detected in human breast milk. The clinical significance of this has not been determined and there is limited experience of nursing women using mesalamine. Caution should be exercised if LIALDA is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of LIALDA tablets in pediatric patients have not been established.
8.5 Geriatric Use
Clinical trials of LIALDA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Systemic exposures are increased in elderly subjects. [see Clinical Pharmacology (12.3)] In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy.
Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia in patients who were 65 years or older who were taking mesalamine-containing products such as LIALDA. Caution should be taken to closely monitor blood cell counts during mesalamine therapy. [see Warnings and Precautions (5.1)]
10 OVERDOSAGE
LIALDA is an aminosalicylate, and symptoms of salicylate toxicity may include tinnitus, vertigo, headache, confusion, drowsiness, sweating, seizures, hyperventilation, dyspnea, vomiting, and diarrhea. Severe intoxication may lead to disruption of electrolyte balance and blood-pH, hyperthermia, dehydration, and end organ damage.
There is no specific known antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage. Fluid and electrolyte imbalance should be corrected by the administration of appropriate intravenous therapy. Adequate renal function should be maintained.
11 DESCRIPTION
Each LIALDA delayed release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:
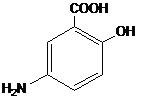
Molecular formula: C7H7NO3
Molecular weight: 153.14
The LIALDA tablet contains 1.2 g mesalamine (5-aminosalicylic acid) in an MMX Multi Matrix System® core of hydrophilic and lipophilic excipients. The MMX® core is coated with a gastro-resistant film of methacrylic acid copolymers, Type A and Type B, which delays mesalamine release until exposure to a pH of 6 and 7, respectively. Upon disintegration of the coating, the core matrix forms a hydrogel and provides extended release of mesalamine across the pH range of 6.8 to 7.2.
The inactive ingredients of LIALDA tablets are sodium carboxymethylcellulose, carnauba wax, stearic acid, silica (colloidal hydrated), sodium starch glycolate (type A), talc, magnesium stearate, methacrylic acid copolymer types A and B, triethylcitrate, titanium dioxide, red ferric oxide and polyethylene glycol 6000.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
Mesalamine has the potential to inhibit the activation of nuclear factor kappa B (NFkB) and consequently the production of key pro-inflammatory cytokines. It has been proposed that reduced expression of PPARy nuclear receptors (y-form of peroxisome proliferator-activated receptors) may be implicated in ulcerative colitis. There is substantial evidence that mesalamine produces pharmacodynamic effects through direct activation of PPARy receptors in the colonic/rectal epithelium.
12.2 Pharmacodynamics
The pharmacodynamic actions of mesalamine occur in the colonic/rectal mucosae local to the delivery of drug from LIALDA into the lumen. There is information suggesting that severity of colonic inflammation in ulcerative colitis patients treated with mesalamine is inversely correlated with mucosal concentrations of mesalamine. However, plasma concentrations representing systemically absorbed mesalamine are not believed to contribute extensively to efficacy.
12.3 Pharmacokinetics
Absorption
The total absorption of mesalamine from LIALDA 2.4 g or 4.8 g given once daily for 14 days to healthy volunteers was found to be approximately 21-22% of the administered dose.
Gamma-scintigraphy studies have shown that a single dose of LIALDA 1.2 g (one tablet) passed intact through the upper gastrointestinal tract of fasted healthy volunteers. Scintigraphic images showed a trail of radio-labeled tracer in the colon, suggesting that mesalamine had distributed throughout this region of the gastrointestinal tract.
In a single dose study, LIALDA 1.2 g, 2.4 g and 4.8 g were administered in the fasted state to healthy subjects. Plasma concentrations of mesalamine were detectable after 2 hours and reached a maximum by 9-12 hours on average for the doses studied. The pharmacokinetic parameters are highly variable among subjects (Table 2). Mesalamine systemic exposure in terms of area under the plasma concentration-time curve (AUC) was slightly more than dose proportional between 1.2 g and 4.8 g LIALDA. Maximum plasma concentrations (Cmax) of mesalamine increased approximately dose proportionately between 1.2 g and 2.4 g and sub-proportionately between 2.4 g and 4.8 g LIALDA, with the dose normalized value at 4.8 g representing, on average, 74% of that at 2.4 g based on geometric means.
Table 2: Mean (SD) PK Parameters for Mesalamine Following Single Dose Administration of LIALDA Under Fasting Conditions
| Parameter1 of Mesalamine | LIALDA 1.2 g
(N=47) | LIALDA 2.4 g
(N=48) | LIALDA 4.8 g
(N=48) |
|---|---|---|---|
| AUC0-t (ng.h/mL) | 9039+ (5054) | 20538 (12980) | 41434 (26640) |
| AUC0-∞ (ng.h/mL) | 9578*** (5214) | 21084 (13185) | 44775# (30302) |
| Cmax (ng/mL) | 857 (638) | 1595 (1484) | 2154 (1140) |
| Tmax* (h) | 9.0**(4.0-32.1) | 12.0 (4.0-34.1) | 12.0 (4.0-34.0) |
| Tlag* (h) | 2.0** (0-8.0) | 2.0 (1.0-4.0) | 2.0 (1.0-4.0) |
| T1/2 (h) (Terminal Phase) | 8.56*** (6.38) | 7.05§ (5.54) | 7.25# (8.32) |
1 Arithmetic mean of parameter values are presented except for Tmax and Tlag.
*Median (min, max); +N=43, ***N=27, §N=33, #N=36, **N=46
Administration of a single dose of LIALDA 4.8 g with a high fat meal resulted in further delay in absorption, and plasma concentrations of mesalamine were detectable 4 hours following dosing. However, a high fat meal increased systemic exposure of mesalamine (mean Cmax: ↑ 91%; mean AUC: ↑ 16%) compared to results in the fasted state. LIALDA was administered with food in the Phase 3 trials.
In a single and multiple dose pharmacokinetic study of LIALDA, 2.4 g or 4.8 g was administered once daily with standard meals to 28 healthy volunteers per dose group. Plasma concentrations of mesalamine were detectable after 4 hours and were maximal by 8 hours after the single dose. Steady state was achieved generally by 2 days after dosing. Mean AUC at steady state was only modestly greater (1.1- to 1.4-fold) than predictable from single dose pharmacokinetics.
In a single dose pharmacokinetic study of LIALDA, 4.8 g was administered in the fasted state to 71 healthy male and female volunteers (28 young (18-35yrs); 28 elderly (65-75yrs); 15 elderly (>75yrs)). Increased age resulted in increased systemic exposure (approximately 2-fold in Cmax), to mesalamine and its metabolite N-acetyl-5-aminosalicylic acid. Increased age resulted in a slower apparent elimination of mesalamine, though there was high between-subject variability. Systemic exposures in individual subjects were inversely correlated with renal function as assessed by estimated creatinine clearance.
| Parameter of 5-ASA |
Young Subjects (18-35 yrs) (N=28) |
Elderly Subjects (65-75 yrs) (N=28) |
Elderly Subjects (>75 yrs) (N=15) |
|---|---|---|---|
| AUC0-t (ng.h/mL) | 51570 (23870) | 73001 (42608) | 65820 (25283) |
| AUC0-∞ (ng.h/mL) | 58057b (22429) | 89612c (40596) | 63067d (22531) |
| Cmax (ng/mL) | 2243 (1410) | 4999 (4381) | 4832 (4383) |
| tmaxa (h) | 22.0 (5.98 - 48.0) | 12.5 (4.00 - 36.0) | 16.0 (4.00 - 26.0) |
| tlaga (h) | 2.00 (1.00 - 6.00) | 2.00 (1.00 - 4.00) | 2.00 (2.00 - 4.00) |
| t1/2 (h), terminal phase | 5.68b (2.83) | 9.68c (7.47) | 8.67d (5.84) |
| Renal clearance (L/h) | 2.05 (1.33) | 2.04 (1.16) | 2.13 (1.20) |
Arithmetic mean (SD) data are presented, N = Number of subjects
a Median (min-max), bN=15, cN=16, dN=13
Distribution
Mesalamine is approximately 43% bound to plasma proteins at the concentration of 2.5 μg/mL.
Metabolism
The only major metabolite of mesalamine (5-aminosalicylic acid) is N-acetyl-5-aminosalicylic acid. Its formation is brought about by N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, principally by NAT-1.
Elimination
Elimination of mesalamine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid (acetylation). However, there is also limited excretion of the parent drug in urine. Of the approximately 21-22% of the dose absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. The apparent terminal half-lives for mesalamine and its major metabolite after administration of LIALDA 2.4 g and 4.8 g were, on average, 7-9 hours and 8-12 hours, respectively.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week dietary carcinogenicity study in CD-1 mice, mesalamine at doses up to 2500 mg/kg/day was not tumorigenic. This dose is 2.2 times the maximum recommended human dose (based on a body surface area comparison) of LIALDA. Furthermore, in a 104-week dietary carcinogenicity study in Wistar rats, mesalamine up to a dose of 800 mg/kg/day was not tumorigenic. This dose is 1.4 times the recommended human dose (based on a body surface area comparison) of LIALDA.
Mutagenesis
No evidence of mutagenicity was observed in an in vitro Ames test or an in vivo mouse micronucleus test.
Impairment of Fertility
No effects on fertility or reproductive performance were observed in male or female rats at oral doses of mesalamine up to 400 mg/kg/day (0.7 times the maximum recommended human dose based on a body surface area comparison). Semen abnormalities and infertility in men, which have been reported in association with sulfasalazine, have not been seen with other mesalamine products during controlled clinical trials.
13.2 Animal Toxicology and/or Pharmacology
In animal studies with mesalamine, a 13-week oral toxicity study in mice and 13-week and 52-week oral toxicity studies in rats and cynomolgus monkeys have shown the kidney to be the major target organ of mesalamine toxicity. Oral daily doses of 2400 mg/kg in mice and 1150 mg/kg in rats produced renal lesions including granular and hyaline casts, tubular degeneration, tubular dilation, renal infarct, papillary necrosis, tubular necrosis, and interstitial nephritis. In cynomolgus monkeys, oral daily doses of 250 mg/kg or higher produced nephrosis, papillary edema, and interstitial fibrosis.
14 CLINICAL STUDIES
14.1 Active, Mild to Moderate Ulcerative Colitis
Two similarly designed, randomized, double blind, placebo-controlled trials were conducted in 517 adult patients with active, mild to moderate ulcerative colitis. The study population was primarily Caucasian (80%), had a mean age of 42 years (6% age 65 years or older), and was approximately 50% male. Both studies used LIALDA doses of 2.4 g/day and 4.8 g/day administered once daily for 8 weeks except for the 2.4 g/day group in Study 1, which was given in two divided doses (1.2g twice daily). The primary efficacy end-point in both trials was to compare the percentage of patients in remission after 8 weeks of treatment for the LIALDA treatment groups vs placebo. Remission was defined as an Ulcerative Colitis Disease Activity Index (UC-DAI) of ≤ 1, with scores of zero for rectal bleeding and for stool frequency, and a sigmoidoscopy score reduction of 1 point or more from baseline.
In both studies, the LIALDA doses of 2.4 g/day and 4.8 g/day demonstrated superiority over placebo in the primary efficacy endpoint (Table 4). Both LIALDA doses also provided consistent benefit in secondary efficacy parameters, including clinical improvement, treatment failure, clinical remission, and sigmoidoscopic improvement. LIALDA 2.4 g/day and 4.8 g/day had similar efficacy profiles.
| Dose | Study 1
(n=262) n/N (%) | Study 2
(n=255) n/N (%) |
|---|---|---|
| LIALDA 2.4 g/day | 30/88 (34.1) | 34/84 (40.5) |
| LIALDA 4.8 g/day | 26/89 (29.2) | 35/85 (41.2) |
| Placebo | 11/85 (12.9) | 19/86 (22.1) |
16 HOW SUPPLIED/STORAGE AND HANDLING
LIALDA is available as red-brown ellipsoidal film coated tablets containing 1.2 g mesalamine, and debossed on one side imprinted with S476.
NDC 54092-476-12 HDPE Bottle with a child-resistant closure of 120 tablets
Store at room temperature 15°C to 25°C (59°F to 77°F); excursions permitted to 30°C (86°F)
See USP Controlled Room Temperature
17 PATIENT COUNSELING INFORMATION
Instruct patients not to take LIALDA if they have hypersensitivity to salicylates (e.g., aspirin) or other mesalamines.
Doctors should ask their patients to tell them all medications they are taking and if they:
- are allergic to sulfasalazine, salicylates or mesalamine;
- are taking non-steroidal anti-inflammatory drugs (NSAIDs) or other nephrotoxic agents;
- are taking azathioprine, or 6-mercaptopurine;
- experience cramping, abdominal pain, bloody diarrhea, fever, headache or rash;
- have a history of myocarditis or pericarditis;
- have kidney or liver disease;
- have a history of stomach blockage;
- are pregnant, intend to become pregnant or are breast-feeding.
Patients should be instructed to swallow LIALDA tablets whole, taking care not to break the outer coating. The outer coating needs to remain intact so that LIALDA is absorbed properly.
Manufactured for Shire US Inc.,
725 Chesterbrook Blvd., Wayne, PA 19087, USA
© 2010 Shire US Inc.
U.S. Patent No. 6,773,720 by license of Giuliani S.p.A., Milan, Italy
MMX® is a registered trademark of Cosmo Technologies Ltd, Wicklow, Ireland.
MMX Multi Matrix System® is a registered trademark of Cosmo S.p.A., Milan, Italy.
PRINCIPAL DISPLAY PANEL
NDC 54092-476-12 - 1.2g 120 Count Bottle
Sharp
Wellspring

NDC 54092-476-08 Display Carton and Sample Carton
Sharp


| LIALDA
mesalamine tablet, delayed release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022000 | 01/16/2007 | |
| Labeler - Shire US Manufacturing Inc. (964907406) |
| Registrant - Shire US Inc. (622467447) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cosmo Spa | 630431955 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Shire US Manufacturing Inc. | 964907406 | manufacture | |
Revised: 09/2010 Shire US Manufacturing Inc.