CETIRIZINE HYDROCHLORIDE
-
cetirizine hydrochloride solution
Taro Pharmaceuticals U.S.A., Inc.
----------
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
Directions
- use only with enclosed dosing cup
- adults and children 6 years and over: 1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours.
- adults 65 years and older: 1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours.
- children 2 to under 6 years of age: 1/2 teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or 1/2 teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours.
- children under 2 years of age: ask a doctor
- consumers with liver or kidney disease: ask a doctor
Inactive ingredients
bubble gum artificial flavor, glacial acetic acid, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium acetate anhydrous, sucralose
Dosing cup should be washed and left to air dry after each use. Do not use if carton is opened or if imprinted safety seal is torn or missing.
Dist. by:
Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
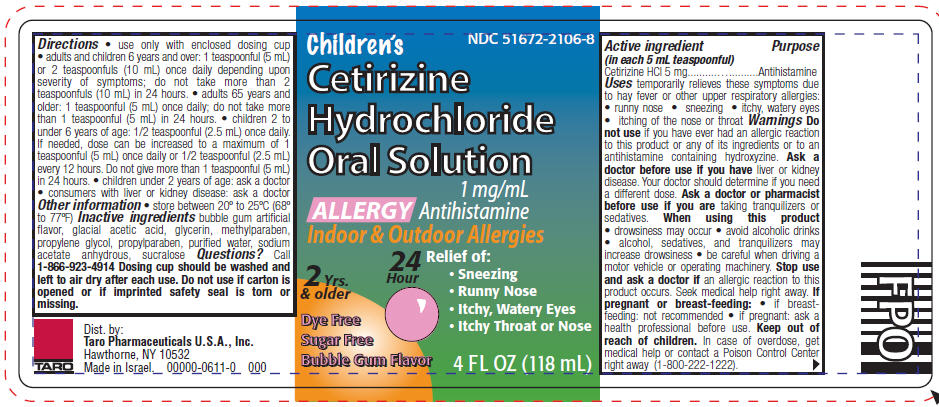
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
NDC 51672-2106-8
Children's
Cetirizine
Hydrochloride
Oral Solution
1 mg/mL
Antihistamine
ALLERGY
Indoor & Outdoor Allergies
2 Yrs.
& older
Dye Free
Sugar Free
Bubble Gum Flavor
24
Hour
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
4 FL OZ (118 mL)
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA201546 | 05/20/2011 | |
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Taro Pharmaceutical Industries, Ltd. | 600072078 | MANUFACTURE | |
Revised: 06/2011 Taro Pharmaceuticals U.S.A., Inc.