GUNA-SHOULDER
-
anti-interleukin-1.alpha. immunoglobulin g rabbit,
canakinumab,
silver nitrate,
metenkefalin,
sus scrofa cartilage,
ferric phosphate,
iris versicolor root,
ranunculus bulbosus,
sanguinaria canadensis root and
sus scrofa tendon injection, solution
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
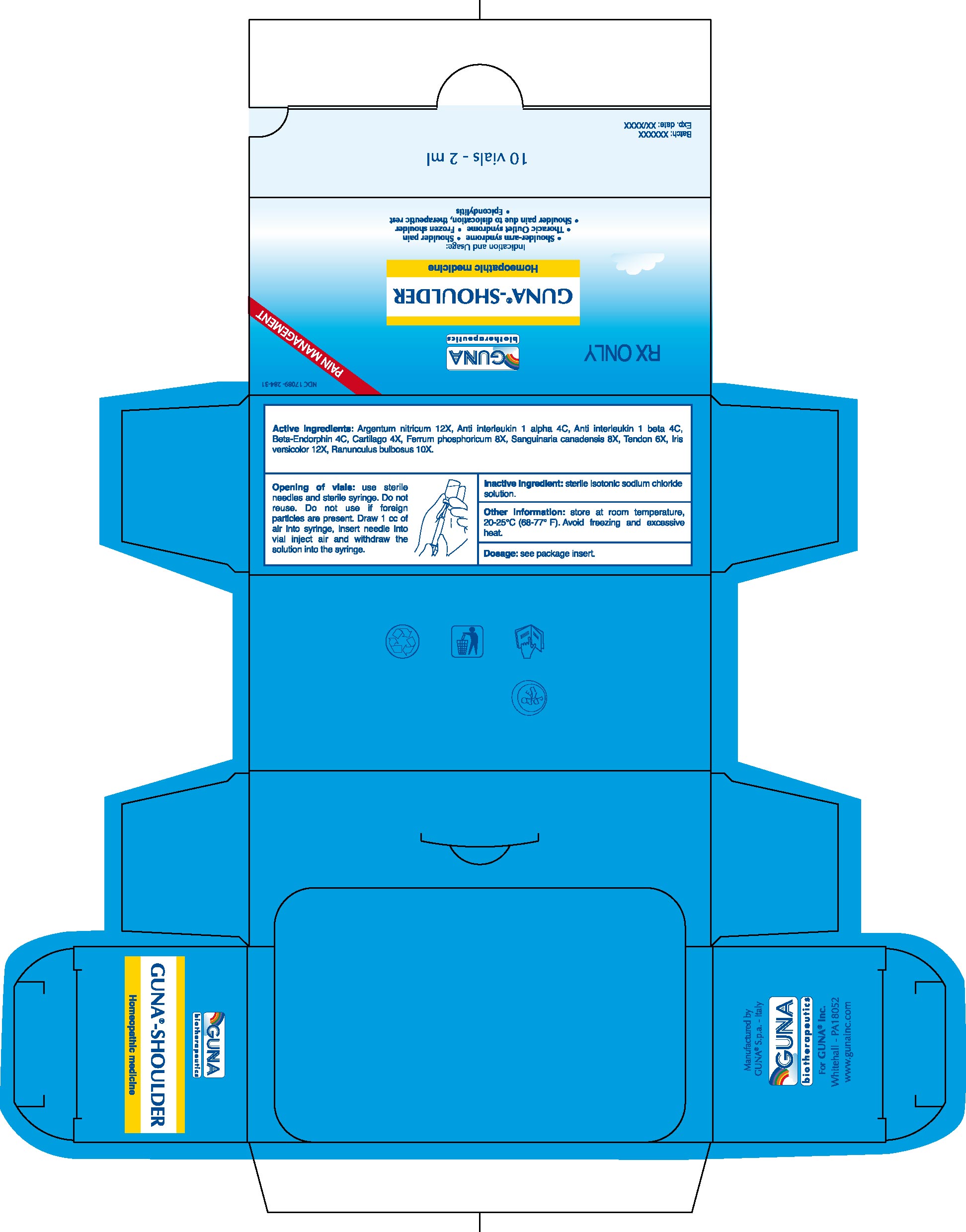
1. INDICATIONS AND USAGE
1.1. Shoulder-arm syndrome
1.2. Shoulder pain
1.3. Thoracic Outlet syndrome (use with GUNA®-NEURAL)
1.4. Frozen shoulder
1.5. Shoulder pain due to dislocation, therapeutic rest
1.6. Epicondylitis.
2. DOSAGE AND ADMINISTRATION
2.1. Standard protocol for IM administration: 1 vial 1-3 times a week according to severity and clinical evolution.
2.2. Standard protocol according to mesotherapy technique using 1 vial per treatment: 2 treatments for the first 2 weeks, 1 treatment a week till pain relief (average 8-10 sessions). For chronic pathologies: continue 1 treatment a week for 1 month till pain relief, then 1 treatment a month.
Select application site according to trigger points, tender points, referred pain zones, acupuncture points, key nerve points, or “local pain points”. Using a 13 mm, 30G or a 4 mm, 27G needle, make the classic intradermal injection according to mesotherapy technique.
Discard unused solution.
2.3. Opening of Vials: Use sterile needles and sterile syringe. Do not reuse. Do not use if foreign particles are present. Draw 1 cc of air into syringe, insert needle into vial, inject air and withdraw the solution
3. DOSAGE FORMS AND STRENGTHS
3.1. 2 ml glass vials
Each ingredient is attenuated according to the procedures stated in the Homeopathic Pharmacopeia of the United States.
Active ingredients: Argentum nitricum 12X, Anti interleukin 1 alpha 4C, Anti interleukin 1 beta 4C, Beta-Endorphin 4C, Cartilago 4X, Ferrum phosphoricum 8X, Sanguinaria canadensis 8X, Tendon 6X, Iris versicolor 12X, Ranunculus bulbosus 10X.
Inactive ingredient: Sterile isotonic sodium chloride solution
4. CONTRAINDICATIONS
4.1. There is no history of hypersensitivity to GUNA®-SHOULDER. However patients with a known hypersensitivity to any ingredient should be tested before use. Make a spot injection (0.1mL) into the forearm and observe for any reactions for 1 hour.
5. WARNINGS AND PRECAUTIONS
5.1. Shoulder pain requires differential diagnosis for chronic cervical syndrome, ischemic heart disease (acute/chronic, on the left side), gallbladder disease (pain on the right side), cervical-brachial nerve pain, muscle trigger points in the trapezius muscles.
5.2. Skin cleansing/disinfection is required before application. Saprophytic bacteria may produce injection site abscesses with improper skin preparation.
6. ADVERSE REACTIONS
6.1 The most common mild adverse reaction is slight reddening at the injection site due to the mechanical effect of the needle or a superficial skin reaction of mild erythema.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA®-SHOULDER. GUNA®- SHOULDER should not be given to a pregnant woman.
8.2 Nursing mothers: It is not known whether any of the ingredients in GUNA®- SHOULDER are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA®- SHOULDER is administered to a nursing woman.
8.3 Pediatric use: No restrictions.
8.4 Geriatric use: No restrictions.
11. DESCRIPTION
11.1 GUNA®-SHOULDER is a sterile solution made with isotonic sodium chloride solution.
It is a homeopathic complex medicine, whose active ingredients have been selected in order to promote 2 main activities:
• Detoxification of the connective tissue matrix
• Pain modulation through stimulation of the physiological mechanism of pain control.
Attenuation of the biological substrates acts to target the area of activity of the product.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Due to the homeopathic nature of the active ingredients, receptors may be activated by feedback regulation. Beta-endorphins at the 4C dose activate the membrane receptors for endogenous endorphins that play a key role in pain relief. Anti IL-1 induces a down regulation of IL-1 inflammatory activity.
12.2. Pharmacodynamics
The physiological effects of GUNA®-SHOULDER are due to the action of the ingredients, as described in the Homeopathic Materia Medica.
In Homeopathy there is no direct relationship between dose and effect, but rather there is a relationship between attenuation and a balancing effect on biochemical pathways.
In GUNA®-SHOULDER the attenuation of each ingredient has been selected according to the Arndt-Schulz Principle (inverted effect law). Attenuation of the physiological ingredients promotes membrane receptor feedback in order to normalize altered biological pathways. In Addition the attenuation technique activates the low dilutions and stabilizes clinical activity of the compound.
12.3. Pharmacokinetics
Homeopathic attenuation provides complete bioavailability of the active ingredients.
13. NONCLINICAL TOXICOLOGY
13.1. GUNA®-SHOULDER has no level of toxicity due to the attenuation of the ingredients.
14. CLINICAL STUDIES
GUNA®-SHOULDER formulation is based on classical Homeopathy and each ingredient has been selected according to its description in the Homeopathic Materia Medica.
The product is intended for application to target points such as acupuncture points, Weihe points, and key neurological points.
Clinical indications of the key ingredients:
Anti interleukin 1 alpha 4C / Anti interleukin 1 beta 4C:
• Biological classification: Interleukin 1 receptor antagonist (IL-1ra) belongs to the IL-1 family. Endogenous IL-1ra is produced in human autoimmune and chronic inflammatory diseases.
• Etiopathogenesis: It binds to IL-1 receptors in competition with IL-1, but does not elicit intracellular response from this binding. Its key role is counteracting the proinflammatory effects of IL-1.
• Space-time localization: next to Arnica. Reference group: Arnica-Mercurius.
• Clinical: Immunological based diseases, autoimmune and chronic inflammatory diseases, acute and chronic pain, osteoarthritis, chronic arthritis, inflammatory psoriasis, wet eczema, localised inflammatory swelling. Appropriate for local application.
• Modalities: Worsens with cold and movement. Improves with rest and warmth.
• Association with other cell mediators: TNF 15C / IL8 4C /
NT4 4C / GCSF 4C.
Beta-Endorphin 4C:
• Biological classification: Neuropeptide and neurotransmitter. It is produced by the anterior lobe of the hypophysis and by the hypothalamus.
• Etiopathogenesis: It acts on the mechanism that enhances pain. It suppresses the memory of painful events and negative experiences.
• Space-time localization: next to Arnica.
Reference group: Mercurius.
• Clinical: Pain management by enhancing the immune response. It acts on modulating pain, cardiac, gastric and vascular function as well as panic syndrome and satiation. Organic and functional pain. Remedy for somatization disorders. It enhances acupuncture sessions. Antidepressive activity. It may improve individual positive attitudes.
• Modalities: Worsens with fatigue and intensive exercise.
• Association with other cell mediators: Sepia / Arnica / Aconitum / Bromum / Aurum / Iodium / IL6 4CH / Melatonin 15C, 30C, 12LM, 18LM, 30LM / NT4 4C / BDNF 4C
15. REFERENCES
15.1. I. Bianchi: Citochine e Interferoni. Farmacologia e Clinica. Nuova IPSA Editore.
15.2. L. Milani: Weihe e altri Punti tra Agopuntura e Omeopatia. Guna Editore.
15.3. J. Malzac: Materia Medica Immunologia. IPSA Editore.
15.4. H.H. Reckeweg: Homeopathic Materia medica. Aurelia Verlag.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. NDC 17089- 284-31 10 glass vials packaged in carton box.16.2. NDC 27089-284-32 50 glass vials packaged in carton box.
16.3. Store at room temperature, 20-25°C (68-77° F). Avoid freezing and excessive heat.
17. PATIENT COUNSELING INFORMATION
17.1. Patients should be informed about Homeopathy and Acupuncture and the main differences with conventional clinical approaches.
| GUNA-SHOULDER
canakinumab - ferric phosphate - iris versicolor root - metenkefalin - ranunculus bulbosus - sanguinaria canadensis root - silver nitrate - sus scrofa cartilage - sus scrofa tendon - anti-interleukin-1.alpha. immunoglobulin g rabbit - injection, solution |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved homeopathic | 09/29/2006 | 06/23/2011 | |
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Guna spa | 430538264 | manufacture | |
Revised: 06/2011 Guna spa