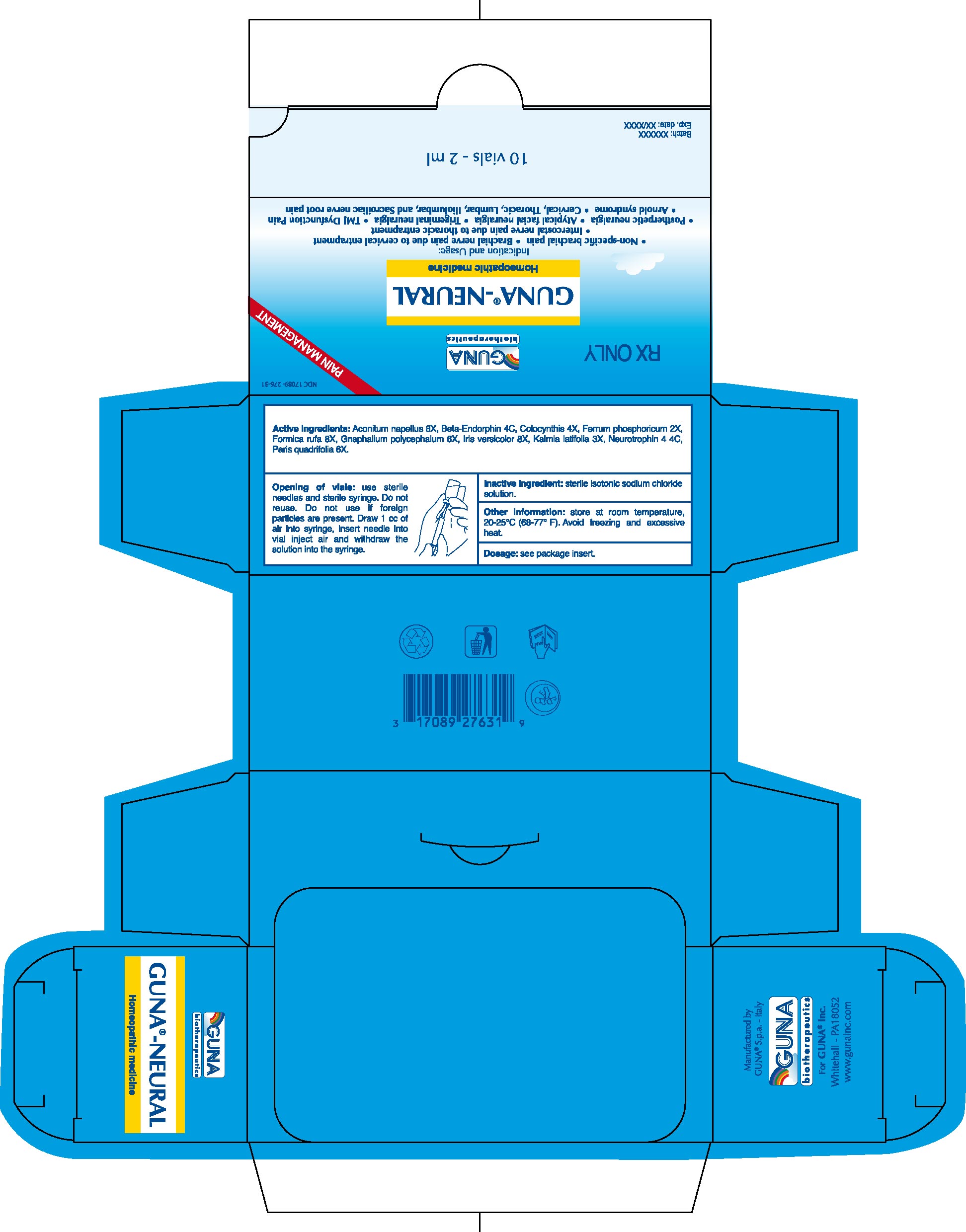
GUNA-NEURAL
-
aconitum napellus,
metenkefalin,
citrullus colocynthis fruit pulp,
ferric phosphate,
formica rufa,
pseudognaphalium obtusifolium,
iris versicolor root,
kalmia latifolia leaf,
neurotrophin-4 and
paris quadrifolia injection, solution
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
1. INDICATIONS AND USAGE
1.1. Non-specific brachial pain
1.2. Brachial nerve pain due to cervical entrapment (use with GUNA®-NECK)
1.3. Intercostal nerve pain due to thoracic entrapment (use with GUNA®-THORACIC)
1.4. Postherpetic neuralgia
1.5. Atypical facial neuralgia(Sluder syndrome, Charlin syndrome)
1.6. Trigeminal neuralgia
1.7. TMJ Dysfunction Pain
1.8. Arnold syndrome (use with GUNA®-NECK)
1.9. Cervical, Thoracic, Lumbar, Iliolumbar, and Sacroiliac nerve root pain (use with GUNA®-NECK, GUNA®-THORACIC, GUNA®-LUMBAR, GUNA®-ISCHIAL, respectively).
2. DOSAGE AND ADMINISTRATION
2.1. Standard protocol for IM administration: 1 vial 1-3 times a week according to severity and clinical progress.
2.2. Standard protocol according to mesotherapy technique: 1 vial per treatment; 2 treatments for the first 2 weeks, 1 treatment a week till pain relief (average 8-10 sessions). For chronic pathologies: continue 1 treatment a week for 1 month until pain relief, then 1 treatment a month. Select application site according to trigger points, tender points, referred pain zones, acupuncture points, nerve key points, Head zones or “local pain points”. Using a 13 mm, 30G or a 4 mm, 27G needle, make the classic intradermal injection according to mesotherapyic technique. Discard unused solution.
2.3. Opening of Vials: Use sterile needles and sterile syringe. Do not reuse. Do not use if foreign particles are present. Draw 1 cc of air into syringe, insert needle into vial inject air and withdraw the solution.
3. DOSAGE FORMS AND STRENGTHS
3.1. 2 ml glass vials
Each ingredient is attenuated according to the Homeopathic Pharmacopeia of the United States.
Active ingredients: Aconitum napellus 8X, Beta-Endorphin 4C, Colocynthis 4X, Ferrum phosphoricum 2X, Formica rufa 8X, Gnaphalium polycephalum 6X, Iris versicolor 8X, Kalmia latifolia 3X, Neurotrophin 4 4C, Paris quadrifolia 6X.
Inactive ingredient: Sterile isotonic sodium chloride solution.
4. CONTRAINDICATIONS
4.1. There is no history of hypersensitivity to GUNA®-NEURAL. However patients with a known hypersensitivity to any ingredient should be tested before use, making a spot injection into one arm and be kept under observation for 1 hour.
5. WARNINGS AND PRECAUTIONS
5.1. Nerve pain requires differential diagnosis for visceral pain, and primary or metastatic cancer pain.
5.2. Skin cleansing/disinfection is required before application. Saprophytic bacteria may produce injection site abscesses with improper skin preparation.
5.3. Patients with known hypersensitivity to formic acid should be kept under observation during the therapy.
6. ADVERSE REACTIONS
6.1. The most common mild adverse reaction is slight reddening at the injection site due to the mechanical effect of the needle or a superficial skin reaction of mild erythema.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA®-NEURAL. GUNA®-NEURAL should not be given to a pregnant woman.
8.2 Nursing mothers: It is not known whether any of the ingredients in GUNA®-NEURAL are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA®-NEURAL is administered to a nursing woman.
8.3 Pediatric use: No restrictions.
8.4 Geriatric use: No restrictions.
11. DESCRIPTION
GUNA®-NEURAL is a sterile solution made with isotonic sodium chloride solution.
It is a homeopathic complex medicine, whose active ingredients have been selected in order to promote 2 main activities:
• Detoxification of the connective tissue matrix
• Pain modulation through stimulation of the physiological mechanism of pain control.
Attenuation of the biological substrates acts to target the area of activity of the product.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Due to the homeopathic nature of the active ingredients, receptors may be activated by feedback regulation. Beta-endorphins at the 4C dose activate the membrane receptors for endogenous endorphins that play a key role in pain relief.
12.2. Pharmacodynamics
The physiological effects of GUNA®-NEURAL are due to the action of the ingredients, as described in the Homeopathic Materia Medica.
In Homeopathy there is no direct relationship between dose and effect, but rather there is a relationship between attenuation and balancing effect on biochemical pathways.
In GUNA®-NEURAL the attenuation of each ingredient has been selected according to the Arndt-Schulz Principle (inverted effect law). membrane receptor feedback in order to normalize altered biological pathways. In Addition the attenuation technique activates the low dilutions and stabilizes clinical activity of the compound.
12.3. Pharmacokinetics
The homeopathic attenuation provides complete bioavailability of the active ingredients.
13. NONCLINICAL TOXICOLOGY
13.1. GUNA®-NEURAL has no level of toxicity due to the attenuation of the ingredients.
14. CLINICAL STUDIES
GUNA®-NEURAL formulation is based on classical Homeopathy and each ingredient has been selected according to its description in the Homeopathic Materia Medica. The product is intended for application to target points such as acupuncture points, Weihe points, and key neurological points.
Clinical indications of the key ingredients:
Beta-Endorphin 4C:
• Biological classification: Neuropeptide and neurotransmitter. It is produced by the anterior lobe of the hypophysis and by the hypothalamus.
• Etiopathogenesis: It acts on the mechanism that enhances pain. It suppresses the memory of painful events and negative experiences.
• Space-time localization: Next to Arnica.
Reference group: Mercurius.
• Clinical: Pain management by enhancing the immune response. It acts on modulating pain, cardiac, gastric and vascular function as well as panic syndrome and satiation. Organic and functional pain. Remedy for somatization disorders. It enhances acupuncture sessions. Antidepressive activity. It may improve individual positive attitudes.
• Modalities: Worsens due to fatigue and intensive exercise.
• Association with other cell mediators: Sepia / Arnica / Aconitum / Bromum / Aurum / Iodium / IL6 4C / Melatonin 15C, 30C, 12LM, 18LM, 30LM / NT4 4C / BDNF 4C.
15. REFERENCES
15.1. L. Milani: Weihe e altri Punti tra Agopuntura e Omeopatia. Guna Editore,
15.2. J. Malzac: Materia Medica Immunologia. IPSA Editore,
15.3. H.H. Reckeweg: Homeopathic Materia medica. Aurelia Verlag.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. NDC 17089-276-31 10 glass vials packaged in carton box.16.2. NDC 17089-276-32 50 glass vials packaged in carton box.
16.3. Store at room temperature, 20-25°C (68-77° F). Avoid freezing and excessive heat.
17. PATIENT COUNSELING INFORMATION
17.1. Patients should be informed about Homeopathy and Acupuncture and the main differences with conventional clinical approaches.
| GUNA-NEURAL
aconitum napellus - ferric phosphate - formica rufa - iris versicolor root - kalmia latifolia leaf - metenkefalin - neurotrophin-4 - paris quadrifolia - pseudognaphalium obtusifolium - citrullus colocynthis fruit pulp - injection, solution |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved homeopathic | 09/29/2006 | 06/22/2011 | |
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Guna spa | 430538264 | manufacture | |
Revised: 06/2011 Guna spa