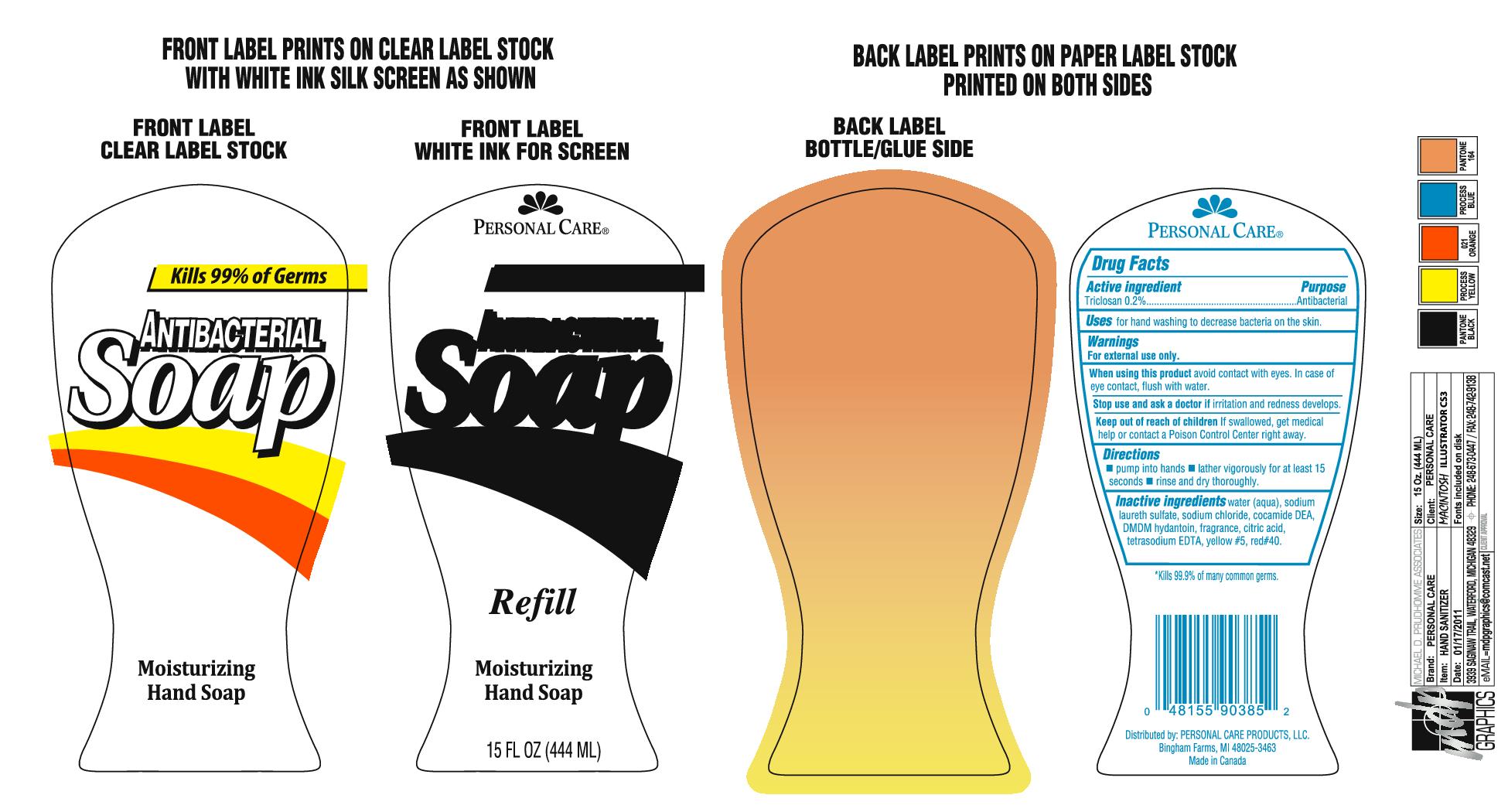
ANTIBACTERIAL
-
triclosan soap
Personal Care Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Keep out of reach of children If swallowed, get medical help or contact a Poison Control Center right away.
Warnings
For external use only.
When using this product avoid contact with eyes. In case of eye contact, flush with water.
Stop use and ask a doctor if irritation and redness develops.
Inactive ingredients
Water (aqua), sodium laureth sulfate, sodium chloride, coamide DEA, DMDM hydantoin, fragrance, citric acid, tetrasodium EDTA, yellow #5, red #40
| ANTIBACTERIAL
triclosan soap |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333A | 01/01/2011 | |
| Labeler - Personal Care Products LLC (966155082) |
| Registrant - Personal Care Products LLC (966155082) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ningbo Pulisi Daily Chemical Products | 529047265 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ningbo United Group Co Ltd | 528196956 | manufacture | |
Revised: 06/2011 Personal Care Products LLC