CLE DE PEAU BEAUTE REFRESHING PROTECTIVE I
-
ensulizole,
octinoxate,
octocrylene and
oxybenzone cream
SHISEIDO CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
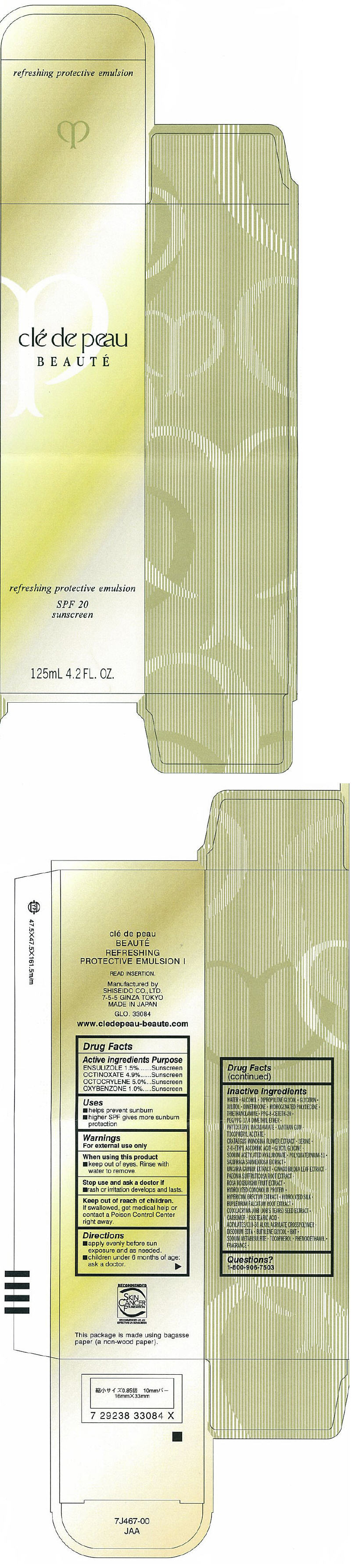
Directions
- apply evenly before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
Inactive ingredients
WATER, ALCOHOL, DIPROPYLENE GLYCOL, GLYCERIN, XYLITOL, DIMETHICONE, HYDROGENATED POLYDECENE, TRIETHANOLAMINE, PPG-8 CETETH-20, PEG/PPG-17/4 DIMETHYL ETHER, PHYTOSTERYL MACADAMIATE, XANTHAN GUM, TOCOPHERYL ACETATE, CRATAEGUS MONOGINA FLOWER EXTRACT, SERINE, 2-O-ETHYL ASCORBIC ACID, GLYCYL GLYCINE, SODIUM ACETYLATED HYALURONATE, POLYQUATERNIUM-51, SAXIFRAGA SARMENTOSA EXTRACT, UNCARIA GAMBIR EXTRACT, GINKGO BILOBA LEAF EXTRACT, PAEONIA SUFFRUTICOSA ROOT EXTRACT, ROSA ROXBURGHII FRUIT EXTRACT, HYDROLYZED CONCHIOLIN PROTEIN, HYPERICUM ERECTUM EXTRACT, HYDROLYZED SILK, BUPLEURUM FALCATUM ROOT EXTRACT, COIX LACRYMA-JOBI (JOB'S TEARS) SEED EXTRACT, CARBOMER, ISOSTEARIC ACID, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, DISODIUM EDTA, BUTYLENE GLYCOL, BHT, SODIUM METABISULFITE, TOCOPHEROL, PHENOXYETHANOL, FRAGRANCE
PRINCIPAL DISPLAY PANEL - 125mL Carton
clé de peau
BEAUTÉ
refreshing protective emulsion
SPF 20
sunscreen
125mL 4.2 FL. OZ.
| CLE DE PEAU BEAUTE REFRESHING PROTECTIVE I
ensulizole, octinoxate, octocrylene, and oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part352 | 02/01/2011 | |
| Labeler - SHISEIDO CO., LTD. (690536453) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| SHISEIDO CO., LTD. | 690536453 | MANUFACTURE, ANALYSIS | |
Revised: 06/2011 SHISEIDO CO., LTD.