PREVAGE EYE ULTRA PROTECTION ANTI AGING MOISTURIZER SPF 15
-
octinoxate and
oxybenzone cream
Elizabeth Arden, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DESCRIPTION
This multi-defense eye cream with advanced idebenon technology provides serious environmental protection, all day intensive moisture and encapsulated sunscreens. Helps smooth the appearance of fine lines, crow's feet and crepiness and helps prevent future aging signs. Eyes instantly appear smoother and brighter. Ophthalmologist tested.
INDICATIONS AND USAGE
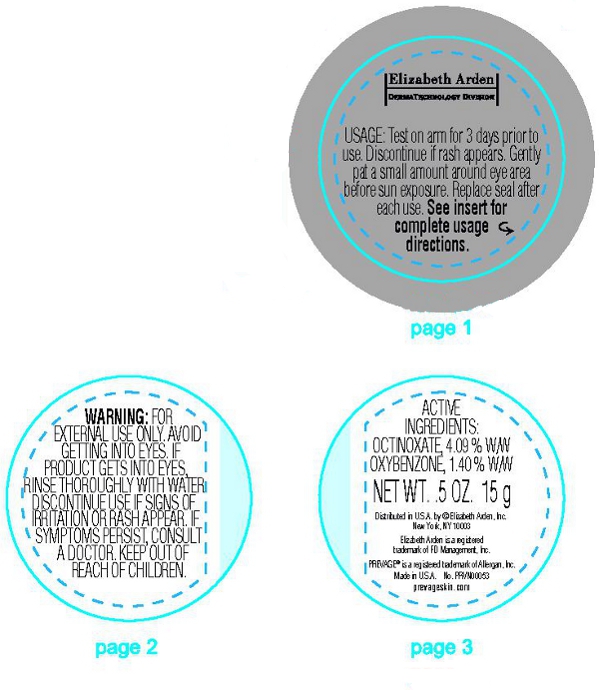
(On Carton) Test Usage: Apply a small amount to your inner arm for three consecutive days. If no reaction occurs, proceed to regular use.
Regular Usage: Gently pat a small amount around eye area before sun exposure. Replace seal after each use. See insert for complete usage directions.
(On Base Label) Usage: Test on arm for 3 days prior to use. Discontinue if rash appears. Gently pat a small amount around eye area before sun exposure. Replace seal after each use. See insert for complete directions.
(On Insert) Test Usage: Skin reactions to PREVAGE products though rare, may be more likely to occur within the first severage days or use or to persons that have sensitive skin, eczema, or a history of allergies. If you have sensitive skin, eczema, or a history of allergies, you may want to consult with your physician before your first use of PREVAGE products. Before beginning to use PREVAGE on a regular basis, apply a small amount of product (less than one pump) daily for at least three (3) days to a small area of skin on the forearm near the inner side of the elbow. If any itching, burning, redness, or swelling occurs during this trial period, wash the product off and discontinue use immediately. If no reactions occur, follow the Regular Usage instructions below.
Regular Usage: Gently pat a small amount around eye area before sun exposure. Replace seal after each use. Avoid getting into eyes. If product gets into eyes, rinse throughly with water.
WARNINGS
Warning: For external use only. Avoid contact with eyes. Discontinue use if signs of iritation or rash appear. If irritation or rash persists, consult a doctor. Keep out of reach of children.
INACTIVE INGREDIENT
Other Ingredients: Water/Aqua/Eau, Butyrospermum Parkii (Shea Butter), Phytosteryl Macadamiate, Glycerin, Butylene Glycol, Dimethicone, Cetearyl Glucoside, Synthetic Beeswax, Theobroma Cacao (Cocoa) Seed Butter, C12-15 Alkyl Benzoate, Stearyl Dimethicone, Dipentaerythrityl Hexacaprylate/Hexacaprate, Tridecyl Trimellitate, Copernicia Cerifera (Canauba) Wax/Cera Carnauba/Cire De Carnauba, Peg-100 Stearate, Hydroxydecyl Ubiquinoyl Dipalmitoyl Glycerate, Beeswax/Cera Alba/Cire D'Abeille, Rabdosia Rubescens Extract, Sigesbeckia Orientalis Extract, Sodium Hyaluronate, Caprylic/Capric Triglyceride, Caprylyl Methicone, BIS-PEG-12 Dimethicone, Caprylyl Glycol, Glyceryl Stearate, Neopentyl Glycol Dicaprylate/Dicaprate, Tridecyl Stearate, Propylene Glycol, Sodium PCA, Trehalose, Urea, Ethylhexylglycerin, Hydrogenated Lecithin, Lecithin, Polyphosphorylcholine Glycol Acrylate, Glycerophosphoinositol Lysine, Acetyl Tetrapeptide-5, Cetearyl Alcohol, PEG-20, Sorbitan Tristearate, Steareth-100, Polyquaternium-51, Sodium Acrylates Copolymer, Hexylene Glycol, Carbomer, PVP, VP/Eicosene Copolymer, Xanthan Gum, Sodium Hydroxide, Disodium EDTA, Mica, Silica, Sodium Citrate, Dimethicone Crosspolymer, Benzoic Acid, Diazolidinyl Urea, Methyparaben, Phenoxyethanol, Potassium Sorbate, Propylparaben, Sorbic Acid, Triacetin, Cetrimonium Chloride, Chlorphenesis, Iron Oxides (CI 77492), Red 40 (CI 16035), Titanium Dioxide (CI 77891).
SPECIAL INSTRUCTIONS
Special Instructions: Skin reactions to PREVAGE products may be more common in individuals who have recently undergone laser resurfacing, dermabrasion, medium to deep chemical peels, or any other aggressive skin therapies. If you have had any such treatments, you should wait 7 to 10 days before starting to us PREVAGE products or consult your physician. If you are using retincids or other skin affecting products dispensed by your physician, ask your physician about the appropriate use of these products in combination with PREVAGE.
| PREVAGE EYE ULTRA PROTECTION ANTI AGING MOISTURIZER SPF 15
octinoxate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part352 | 03/14/2011 | |
| Labeler - Elizabeth Arden, Inc (794337337) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Englewood Lab | 172198223 | manufacture | |
Revised: 01/2011 Elizabeth Arden, Inc