ionsys (fentanyl hydrochloride) patch, extended release, electrically controlled
[Ortho-McNeil, Inc.]
CII
Patient-activated
* Equals to 44.4 mcg fentanyl HCl
IONSYS™ should only be used for the treatment of hospitalized patients. Treatment with IONSYS™ should be discontinued before patients are discharged from the hospital.
Treatment with fentanyl, the active component of IONSYS™, may result in potentially life-threatening respiratory depression and death. To avoid potential overdosing, only the patient should activate IONSYS™ dosing.
Inappropriate use of IONSYS™, leading to ingestion or contact with mucous membranes or unintended exposure to the fentanyl hydrogel could lead to the absorption of a potentially fatal dose of fentanyl. Therefore, the hydrogels should not come into contact with fingers or mouth.
IONSYS™ contains fentanyl, a potent opioid agonist and Schedule II controlled substance with high potential for abuse similar to hydromorphone, methadone, morphine, and oxycodone. Fentanyl can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing IONSYS™ in situations where the Health Care Professional is concerned about an increased risk of misuse, abuse, or diversion. After the maximum dosage administration, a significant amount of fentanyl remains in the device.
IONSYS™ should always be kept out of reach of children.
DESCRIPTION
IONSYS™ (fentanyl iontophoretic transdermal system) is a patient-controlled iontophoretic transdermal system providing on-demand systemic delivery of fentanyl, an opioid agonist, for up to 24 hours or a maximum of 80 doses, whichever comes first.
The chemical name is propanamide, N-phenyl-N-[1-(2-phenylethyl)-4- piperidinyl] monohydrochloride. The structural formula is:
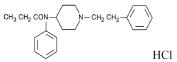
The molecular weight of fentanyl hydrochloride is 372.93, and the empirical formula is C22H28N2O·HCl. The n-octanol:water partition coefficient is 860:1; the pKa is 8.4.
The active ingredient in IONSYS™ is fentanyl HCl, which is equivalent to 40 mcg per dose of fentanyl free base. IONSYS™ contains 10.8 mg of fentanyl hydrochloride equivalent to 9.7 mg of fentanyl. IONSYS™ is designed to deliver a 40-mcg dose of fentanyl (equivalent to 44.4 mcg of fentanyl hydrochloride) over a 10-minute period upon each activation of the dose button (see DOSAGE AND ADMINISTRATION).
The inactive ingredients in the IONSYS™ hydrogels consist of cetylpyridinium chloride, USP; citric acid, USP; polacrilin; polyvinyl alcohol; sodium citrate, USP; sodium chloride, USP; sodium hydroxide; and purified water, USP.
System Components and Structure
Each IONSYS™ system is composed of a plastic top housing that contains the battery and electronics, and a red plastic bottom housing containing two hydrogel reservoirs and a polyisobutylene skin adhesive. Only one of the hydrogels (the anode, located under the dosing button) contains fentanyl HCl, along with inactive ingredients. The other hydrogel (the cathode) contains only pharmacologically inactive ingredients. The bottom housing has a red tab that is used only for system removal from the skin and during disposal (see DOSAGE AND ADMINISTRATION, Disposal). A siliconized clear, plastic release liner covers the hydrogels and must be removed and discarded prior to placement on the skin. The system is powered by a 3-volt lithium battery.
IONSYS™ (fentanyl iontophoretic transdermal system)
CLINICAL PHARMACOLOGY
Pharmacology
Fentanyl is an opioid analgesic. Fentanyl interacts predominantly with the opioid μ-receptor. These μ-binding sites are discretely distributed in the human brain, spinal cord, and other tissues.
In clinical settings, fentanyl exerts its principal pharmacologic effects on the central nervous system. Its primary actions of therapeutic value are analgesia and sedation. Fentanyl may increase the patient's tolerance for pain and decrease the perception of suffering, although the presence of the pain itself may still be recognized.
In addition to analgesia, alterations in mood, euphoria and dysphoria, and drowsiness commonly occur. Fentanyl depresses the respiratory centers and the cough reflex, and constricts the pupils. Analgesic blood concentrations of fentanyl may cause nausea and vomiting by directly stimulating the chemoreceptor trigger zone, but nausea and vomiting are significantly more common in ambulatory than in recumbent patients, as is postural syncope. Opioids increase the tone and decrease the propulsive contractions of the smooth muscle of the gastrointestinal tract. The resultant prolongation in gastrointestinal transit time may be responsible for the constipating effect of opioids. Because opioids may increase biliary tract pressure, some patients with biliary colic may experience worsening rather than relief of pain.
While opioids generally increase the tone of urinary tract smooth muscle, the net effect tends to be variable, in some cases producing urinary urgency, in others, difficulty in urination.
At therapeutic dosages, fentanyl usually does not exert major effects on the cardiovascular system. However, some patients may exhibit orthostatic hypotension and fainting. Histamine assays and skin wheal testing in humans indicate that clinically significant histamine release rarely occurs with fentanyl administration. Assays in humans show no clinically significant histamine release in dosages up to 50 mcg /kg.
Pharmacodynamics
Analgesia
When patients titrated themselves to analgesic effect with IONSYS™ 40 mcg, serum concentrations were in the range of 0.4 to 1.5 ng/mL over the 24-hour dosing period.
Respiratory Effects
Hypoventilation can occur throughout the therapeutic range of fentanyl serum concentrations and may occur at any time during therapy, especially for patients who have an underlying pulmonary condition or who receive doses of opioids or other CNS drugs associated with hypoventilation in addition to IONSYS™. The respiratory effects of IONSYS™ should be monitored by clinical evaluation, including oxygen saturation, respiratory rate, and degree of sedation. After delivery of the maximum number of doses in the shortest possible time period (80 consecutive doses delivered over approximately 13 hours), the fentanyl serum concentration was in the range of 1.51 to 2.37 ng/mL, which is in the range that could result in respiratory depression.
See WARNINGS, PRECAUTIONS and OVERDOSAGE for additional information on respiratory depression.
Cardiovascular Effects
Fentanyl may produce bradycardia.
CNS Effects
Central nervous system effects, such as sedation and depression of respiration, increase with increasing serum fentanyl concentrations.
Pharmacokinetics
Unless otherwise specified, the clinical pharmacology studies described in this section were performed in healthy adult volunteers. Volunteers were administered naltrexone to antagonize the opioid effects of fentanyl.
Absorption
At the initiation of each dose, an electrical current is activated, which moves a dose of fentanyl from the drug-containing reservoir through the skin and into the systemic circulation. Compared to IV fentanyl administration, fentanyl concentrations in blood increase slowly with IONSYS™ activation and continue to increase for approximately 5 minutes after the completion of each 10-minute dose.
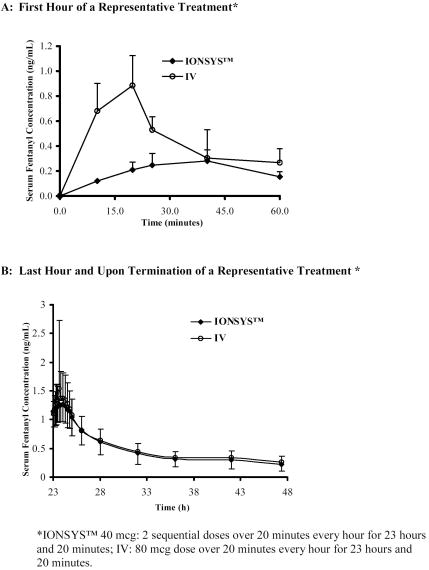
The systemic absorption of fentanyl from IONSYS™ increases as a function of time, and this increase appears to be independent of frequency of dosing. When a dose of 40 mcg is administered at treatment initiation, the amount of fentanyl absorbed is expected to be approximately 16 mcg (see Figures 1A, 1B). In clinical pharmacokinetic studies, on-demand dosing was initiated immediately after IONSYS™ application. This resulted in absorption of a 40 mcg fentanyl dose by about 10 hours post treatment initiation. Thereafter, a 40-mcg dose of fentanyl is delivered with each activation.
After delivery of the maximum number of doses in the shortest possible time period (80 consecutive doses delivered over approximately 13 hours), the average maximum fentanyl serum concentration was 1.94 ± 0.43 ng/mL. Pharmacokinetic data from illustrative dosing regimens are represented in Table 1. When IONSYS™ was applied without activating the current, the average absorption rate for fentanyl over 24 hours was 2.3 mcg/h.
Inter-subject variability in fentanyl AUC following IONSYS™ treatment (33%) was comparable to IV fentanyl treatment (28%), suggesting that the iontophoretic transdermal system does not add to the inherent between-subject variability in fentanyl AUC.
The delivery of fentanyl from IONSYS™ is similar whether applied on the upper outer arm or the chest. When the system is placed on the lower inner arm, the delivery of fentanyl is approximately 20% lower. Other application sites have not been evaluated.
| Dosing Regimen | ||
|
*AUC for this dosing regimen is value estimated between 23-24 hours |
||
|
**Average AUC over all doses delivered during the treatment duration (13.33 hours) |
||
|
a Representative dosing regimen |
||
|
b Maximum theoretical dosing |
||
| Parameter | 48a doses (two sequential doses every hour for 23 hours and 20 minutes) | 80b sequential doses (one dose every ten minutes for 13 hours and 20 minutes) |
| AUC per on-demand dose (ng/mL) | 0.57±0.13* | 0.51±0.16** |
| Cmax
(ng/mL) | 1.3±0.3 | 1.94±0.43 |
Distribution
Fentanyl administered intravenously exhibits a three-compartment disposition model. In healthy volunteers after IV administration, the estimated initial distribution half-life was about 6 minutes; the second distribution half-life was about 1-hour; and the terminal half-life was about 16 hours. The average volume of distribution for fentanyl at steady state following IV administration is 833 L.
Mean values for unbound fractions of fentanyl in plasma are estimated to be between 13 and 21%. Fentanyl binds to erythrocytes, α1-acid glycoproteins, and plasma albumin.
Binding is independent of drug concentration over the therapeutic range. Fentanyl plasma protein binding capacity decreases with increasing ionization of the drug. Alterations in blood pH may alter ionization of fentanyl and therefore its distribution between plasma and the central nervous system. Fentanyl accumulates in the skeletal muscle and fat and is released slowly into the blood.
Metabolism
In humans, fentanyl is metabolized primarily by cytochrome P450 3A4-mediated N-dealkylation to norfentanyl and other inactive metabolites that do not contribute materially to the observed activity of the drug. The average clearance in healthy subjects following IV administration was observed to be 53 L/h. Within 72 hours of IV fentanyl administration, approximately 75% of the dose is excreted in urine, mostly as metabolites, with less than 10% representing unchanged drug. Approximately 9% of the dose is recovered in the feces, primarily as metabolites.
A decline in fentanyl concentration after termination of treatment and the terminal half-life is similar following IV administration of fentanyl and IONSYS™ (see Figure 1B). This suggests a negligible contribution from continued absorption of fentanyl remaining in the skin.
Skin does not metabolize fentanyl administered transdermally. This was determined in a human keratinocyte cell assay.
Drug Interactions
Agents Affecting Cytochrome P450 3A4
Fentanyl is metabolized mainly via the cytochrome P450 3A4 enzyme (CYP3A4). Therefore, drug interactions may occur when IONSYS™ is given concurrently with agents that affect CYP3A4 activity. Coadministration with agents that induce CYP3A4 activity, such as rifampin, carbamazepine, phenytoin, and Saint John's Wort, may cause increased clearance of fentanyl and reduce the efficacy of IONSYS™. The concomitant use of fentanyl with CYP3A4 inhibitors such as macrolide antibiotics (e.g. erythromycin), azole antifungal agents (e.g. ketoconazole), or protease inhibitors (e.g. ritonavir) may result in a decrease in fentanyl clearance, which could increase or prolong adverse drug effects including serious respiratory depression. In this situation, special patient care and observation are appropriate.
Special Populations
Literature suggests that clearance of fentanyl may be reduced and the terminal half-life prolonged in the elderly (see PRECAUTIONS). In a pharmacokinetic study conducted in 63 healthy volunteers where several demographic factors (age, race, gender, and body mass index) were evaluated, none of these factors had a significant effect on the extent of drug absorption (AUC) following administration of IONSYS™.
CLINICAL STUDIES
Placebo-controlled Trials
The efficacy and safety of IONSYS™ for treatment of short-term acute pain were evaluated in three placebo-controlled studies in postoperative patients. The patients were predominantly female (70-83%) and Caucasian (79-84%), and their mean age was 45-54 years (range, 18-90 years). Patients were enrolled while in the recovery room shortly after major surgery (predominantly lower abdominal or orthopedic) if they were expected to require at least 24 hours of parenteral opioid treatment and were not opioid tolerant; their ASA (American Society of Anesthesiologists) physical status was I, II, or III; and their postsurgical recovery was expected to be uncomplicated. Across the trials, 154 patients were ASA I status (21%); 435 patients were ASA II status (60%); and 138 patients were ASA III status (19%). Administration of long-lasting or continuous regional analgesics, or any non-opioid analgesics was not permitted in the studies. Patients who remained in the studies for three or more hours using IONSYS™ (or the control) for patient-controlled analgesia (PCA) were considered evaluable.
In the immediate postoperative period, patients were titrated to comfort with IV fentanyl or morphine per hospital protocol. Once comfortable, patients were randomized and the IONSYS™ or matching placebo system was applied. Patients were instructed to use the system for pain relief. Supplemental IV fentanyl was administered by bolus injection as needed to achieve comfort up to three hours post-enrollment. The percentage of patients who used rescue medication during these three hours, as well as the mean amount of rescue medication used, is shown in Table 2 below.
| IONSYS™ | Placebo | |
| Study 1 | 45 % (83 mcg) | 52 % (102 mcg) |
| Study 2 | 48 % (100 mcg) | 55 % (95 mcg) |
| Study 3 | 34 % (78 mcg) | 36 % (76 mcg) |
After Study Hour 3, IONSYS™ alone or the placebo treatment alone was used to provide analgesia.
In each of the three randomized, double-blind, placebo-controlled trials, fewer patients discontinued for lack of efficacy from three hours to twenty-four hours after IONSYS™ application (see Table 3).
| Study | IONSYS™ n=454 | Placebo n=273 | p-value |
| Study 1 | 27 % (64/235) | 57 % (116/204) | <0.0001 |
| Study 2 | 25 % (36/142) | 40 % (19/47) | 0.049 |
| Study 3 | 8 % (6/77) | 41 % (9/22) | 0.0001 |
The last mean pain intensity scores recorded during the 24-hour treatment period are presented in Table 4.
| Study | IONSYS™ n=454 | Placebo n=273 | p-value |
|
a Verbal numerical rating score 0-10 at 24 hours or at discontinuation |
|||
|
b Visual analogue scale, 0-100 mm at 24 hours or at discontinuation |
|||
| Study 1 (NRSa) | 3.4 | 5.3 | <0.0001 |
| Study 2 (VASb) | 31 | 41 | 0.0474 |
| Study 3 (VASb) | 21 | 37 | 0.0006 |
The type of surgical procedure did not influence the trends in efficacy endpoints.
The efficacy of IONSYS™ was similar across the range of body mass indices studied (<25 to≥40 kg/m2 Body Mass Index).
Patients who completed 24 hours of IONSYS™ treatment in the controlled studies used a wide range of the available 80 doses, with a mean of 29 doses/patient (range of 0-93 doses). The majority of patients (56.5%) used between 11 to 50 doses. One percent of patients required a second system within 24 hours, after exhausting the first system.
INDICATIONS AND USAGE
IONSYS™ is indicated for the short-term management of acute postoperative pain in adult patients requiring opioid analgesia during hospitalization. Patients should be titrated to an acceptable level of analgesia before initiating treatment with IONSYS™. IONSYS™ is not intended for home use and is, therefore, inappropriate for use in patients once they have been discharged from the hospital. It is not recommended for patients under the age of 18 years (see WARNINGS and PRECAUTIONS).
CONTRAINDICATIONS
IONSYS™ is contraindicated in patients with known hypersensitivity to fentanyl, cetylpyridinium chloride (e.g. Cepacol®) or any components of the IONSYS™ system.
WARNINGS
IONSYS™ should be prescribed only by persons knowledgeable in the administration of potent opioids and in the management of patients receiving potent opioids for treatment of pain. Patients treated with IONSYS™ should be under the supervision of medical personnel with expertise in the detection and management of hypoventilation, including airway management and the use of opioid antagonists.
Inappropriate use of IONSYS™, leading to ingestion or contact with mucous membranes, or unintended exposure to the fentanyl hydrogel, could lead to the absorption of a potentially fatal dose of fentanyl. Therefore, the hydrogels should not come into contact with fingers or mouth.
The mean terminal elimination half-life of IONSYS™ is 11 hours. Therefore, patients who have experienced serious adverse events, including overdose, will require continued monitoring after IONSYS™ removal since serum fentanyl concentrations decline gradually (See Figure 1B).
To avoid potential overdosing, only the patient should activate IONSYS™. IONSYS™ should be used only in hospitals, by patients under medical supervision and direction. More than one IONSYS™ system should not be applied to a patient at the same time.
If the fentanyl hydrogel becomes separated from the IONSYS™ system, contact can be harmful to humans and animals. If the hydrogel becomes separated from the IONSYS™ system during removal, use gloves or tweezers to remove the hydrogel from the skin and properly dispose of in accordance with state and federal regulations for controlled substances. The skin area that had been in contact with the hydrogel should be thoroughly flushed with water. Do not use soap, alcohol, or other solvents to remove the hydrogel as they may enhance the drug's ability to penetrate the skin. In the event that the IONSYS™ system falls off, ensure that the entire IONSYS™ system (i.e. with hydrogel) is collected and properly disposed of.
Prior to discharge from the hospital, medical personnel must remove the IONSYS™ system and dispose of it properly (see DOSAGE AND ADMINISTRATION, Disposal). After the maximum dosage administration, a significant amount of fentanyl remains in the device.
Misuse, Abuse and Diversion of Opioids
Fentanyl is an opioid agonist of the morphine type. Opioid agonists are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Fentanyl can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing IONSYS™ in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Fentanyl may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
PRECAUTIONS
General
Only patients who are able to understand and follow the instructions to operate IONSYS™ should use the system.
Patients should be titrated to an acceptable level of analgesia before initiating dosing with the IONSYS™ system.
IONSYS™ should be applied to intact, non-irritated, and non-irradiated skin on the chest or upper outer arm. Care should be taken to avoid exposing IONSYS™ to water as this could cause the system to fall off or stop working.
The error detection circuitry in IONSYS™ uses a series of audible signals to alert the patient or caregiver when a dose is not being delivered in response to the patient's attempt to activate a dose. Therefore, IONSYS™ should be used with caution in patients who have high frequency hearing impairment (see DOSAGE AND ADMINISTRATION, Troubleshooting). The testing instructions in the Dosage and Administration section can be used to demonstrate the audible tone for patients if there is any question of the patient's ability to hear the tone.
IONSYS™ contains metal parts and should be removed before an MRI procedure, cardioversion, or defibrillation to avoid damage to the system from the strong electromagnetic fields set up by these procedures. (See DOSAGE AND ADMINISTRATION, Disposal). IONSYS™ contains radio-opaque components and may interfere with an X-ray image or CAT scan. The low-level electrical current provided by IONSYS™ does not result in electromagnetic interference with other electromechanical devices like pacemakers or electrical monitoring equipment.
Interactions with other CNS Depressants
The concomitant use of other central nervous system depressants, including other opioids, sedatives, hypnotics, general anesthetics, phenothiazines, tranquilizers, skeletal muscle relaxants, sedating antihistamines, or alcoholic beverages, may produce additive depressant effects. Hypoventilation, hypotension, profound sedation, respiratory depression, coma, or death may result. Therefore, the use of concomitant CNS depressants requires individual adjustment of dosage of the concomitant medication and close observation of the patient.
As with other opioid medications, IONSYS™ may impair the mental and/or physical ability required for the performance of potentially hazardous tasks.
Chronic Opioid Therapy
IONSYS™ has not been studied in the treatment of breakthrough pain in patients on chronic opioid therapy and is not recommended for this purpose.
Patients on chronic opioid therapy or with a history of opioid abuse may require higher analgesic doses in the post-operative period than are available from IONSYS™; therefore these patients should be evaluated frequently to ensure they are receiving adequate analgesia.
Topical Skin Reactions
Topical skin reactions (erythema, sweating, vesicles, papules/pustules) may occur after removal of IONSYS™ and are typically limited to the application site area. Reactions typically resolve without treatment. If a severe skin reaction is observed, treat with a topical antiseptic/antibiotic as appropriate.
Respiratory Depression
Fentanyl may cause potentially life-threatening respiratory depression. Consequently, patients with hypoventilation should be carefully observed for degree of sedation and their respiratory rate monitored until respiration has stabilized.
The use of concomitant CNS-active drugs requires special patient care and observation (see WARNINGS).
Pulmonary Disease
Because potent opioids may cause serious or life-threatening hypoventilation, IONSYS™ should be administered with caution to patients with pre-existing medical conditions predisposing them to hypoventilation. In such patients, analgesic doses of opioids may further decrease respiratory drive to the point of respiratory failure.
Head Injuries and Increased Intracranial Pressure
IONSYS™ should not be used in patients who may be particularly susceptible to the intracranial effects of CO2 retention, such as those with evidence of increased intracranial pressure, impaired consciousness, or coma. Opioids may obscure the clinical course of patients with head injury. IONSYS™ should be used with caution in patients with brain tumors.
Cardiac Disease
Fentanyl may produce bradycardia in some patients. Therefore, IONSYS™ should be administered with caution to patients with bradyarrhythmias.
Hepatic Disease
Insufficient data are available on the use of IONSYS™ in patients with impaired hepatic function. Since fentanyl is eliminated by hepatic metabolism, and fentanyl clearance may decrease in patients with hepatic disease, IONSYS™ should be used with caution in these patients.
Renal Disease
Approximately 10% of administered fentanyl is excreted unchanged by the kidney. Insufficient data are available on the use of IONSYSTM in patients with impaired renal function. IONSYS™ should be used with caution in these patients.
Patient Information
A Patient Instructions for Use sheet is included in the package for IONSYS™ (see Patient Bedside Information Sheet). Health care professionals are encouraged to review this material with patients.
- Patients should be advised not to let anyone else activate the dosing button on the IONSYS™ system since only the patient knows how much pain he or she is experiencing. Patients should be cautioned that allowing others to activate the device may result in a potentially fatal overdose.
- Patients should be instructed not to touch the sticky side of the system and not to touch the gels. Patients should be cautioned that fentanyl is rapidly absorbed by the eyes and mouth, and could be harmful or fatal if absorbed this way. Patients should be advised to inform a health care provider if accidental exposure occurs and to immediately rinse the affected area with copious amounts of water. Soap, alcohol, or other solvents should not be used because they may enhance permeability.
- Patients should be advised to inform the health care provider of any allergies to fentanyl, cetylpiridinium chloride (e.g. Cepacol®), or any components of the IONSYS™ system.
- Patients should be advised not to give IONSYS™ to other people, as it may lead to serious and life-threatening events.
Drug Interactions
Central Nervous System Depressants
The concomitant use of other central nervous system depressants, including other opioids, sedatives, hypnotics, general anesthetics, phenothiazines, tranquilizers, skeletal muscle relaxants, sedating antihistamines, or alcoholic beverages, may produce additive depressant effects. Hypoventilation, hypotension, profound sedation, coma, or death may occur. Therefore, use of concomitant CNS depressants requires individual adjustment of dosage of the concomitant medication and observation of a given patient.
Agents Affecting CYP3A4 Isoenzyme System
Fentanyl is metabolized mainly via the cytochrome P450 3A4 enzyme (CYP3A4). Therefore, drug interactions may occur when IONSYS™ is given concurrently with agents that affect CYP3A4 activity. Coadministration with agents that induce CYP3A4 activity, such as rifampin, carbamazepine, phenytoin, and Saint John's Wort may cause increased clearance of fentanyl and reduce the efficacy of IONSYS™. The concomitant use of fentanyl with CYP3A4 inhibitors such as macrolide antibiotics (e.g. erythromycin), azole antifungal agents (e.g. ketoconazole), or protease inhibitors (e.g. ritonavir) may result in a decrease in fentanyl clearance, which could increase or prolong adverse drug effects including serious respiratory depression. In this situation, special patient care and observation are appropriate.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Studies in animals to evaluate the carcinogenic potential of fentanyl HCl have not been conducted. There was no evidence of mutagenicity in the Ames Salmonella mutagenicity assay, the primary rat hepatocyte unscheduled DNA synthesis assay, the BALB/c 3T3 transformation test, and the human lymphocyte and CHO chromosomal aberration in-vitro assays.
The potential effects of fentanyl on male and female fertility were examined in the rat model via two separate experiments. In the male fertility study, male rats were treated with fentanyl (0, 0.025, 0.1 or 0.4 mg/kg/day) via continuous intravenous infusion for 28 days prior to mating; female rats were not treated. In the female fertility study, female rats were treated with fentanyl (0, 0.025, 0.1 or 0.4 mg/kg/day) via continuous intravenous infusion for 14 days prior to mating until day 16 of pregnancy; male rats were not treated. Analysis of fertility parameters in both studies indicated that an intravenous dose of fentanyl up to 0.4 mg/kg/day to either the male or the female alone produced no effects on fertility (this dose is approximately 1.2 times the maximum available daily human dose on a mg/m2 basis). In a separate study, a single daily bolus dose of fentanyl was shown to impair fertility in rats when given in intravenous doses of 0.3 times the human dose for a period of 12 days.
Pregnancy
Teratogenic Effects—Pregnancy Category C
No epidemiologic studies of congenital anomalies in infants born to women treated with fentanyl during pregnancy have been reported.
The potential effects of fentanyl on embryo-fetal development were studied in the rat, mouse, and rabbit models.
Published literature reports that administration of fentanyl (0, 10, 100, or 500 mcg/kg/day) to pregnant female Sprague-Dawley rats from day 7 to 21 via implanted microosmotic minipumps did not produce any evidence of teratogenicity (the high dose is approximately 1.5 times the maximum available daily human dose on a mg/m2 basis).
In contrast, the intravenous administration of fentanyl (0, 0.01, or 0.03 mg/kg) to bred female rats from gestation day 6 to 18 suggested evidence of embryotoxicity and a slight increase in mean delivery time in the 0.03 mg/kg/day group. There was no clear evidence of teratogenicity noted.
Pregnant female New Zealand White rabbits were treated with fentanyl (0, 0.025, 0.1, 0.4 mg/kg) via intravenous infusion from day 6 to day 18 of pregnancy. Fentanyl produced a slight decrease in the body weight of the live fetuses at the high dose, which may be attributed to maternal toxicity. Under the conditions of the assay, there was no evidence for fentanyl induced adverse effects on embryo-fetal development at doses up to 0.4 mg/kg (approximately 3 times the maximum achievable human daily dose on a mg/m2 basis).
There are no adequate and well-controlled studies in pregnant women. IONSYS™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Chronic maternal treatment with fentanyl during pregnancy has been associated with transient respiratory depression, behavioral changes, or seizures characteristic of neonatal abstinence syndrome in newborn infants. Symptoms of neonatal respiratory or neurological depression were no more frequent than expected in most studies of infants born to women treated acutely during labor with intravenous or epidural fentanyl. Transient neonatal muscular rigidity has been observed in infants whose mothers were treated with intravenous fentanyl.
The potential effects of fentanyl on prenatal and postnatal development were examined in the rat model. Female Wistar rats were treated with 0, 0.025, 0.1, or 0.4 mg/kg/day fentanyl via intravenous infusion from day 6 of pregnancy through 3 weeks of lactation. Fentanyl treatment (0.4 mg/kg/day) significantly decreased body weight in male and female pups and also decreased survival in pups at day 4. Both the mid-dose and high-dose of fentanyl animals demonstrated alterations in some physical landmarks of development (delayed incisor eruption and eye opening) and transient behavioral development (decreased locomotor activity at day 28 which recovered by day 50). The mid-dose and the high-dose are 0.3 and 1.5 times the maximum available daily human dose on a mg/m2 basis.
Labor and Delivery
Fentanyl readily passes across the placenta to the fetus; therefore IONSYS™ is not recommended for analgesia during labor and delivery.
Nursing Mothers
Fentanyl is excreted in human milk; therefore IONSYS™ is not recommended for use in nursing women because of the possibility of sedation and/or respiratory depression in their infants.
Pediatric Use
Not for pediatric use. The efficacy and safety of IONSYS™ have not been adequately studied in pediatric patients under 18 years of age.
Preliminary pediatric studies using iontophoretically-delivered fentanyl at a lower dose suggested that pediatric patients were more vulnerable to application site reactions, which were more severe than in adults.
Geriatric Use
IONSYS™ 40 mcg has been studied in 499 patients 65 years or older; 174 of whom were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. However, the incidence of the following events was slightly higher (≥ 1%) in patients ≥ 65 years compared with patients who were 18 to 64 years of age: hypotension (4 % versus 3%), confusion (2% versus <1%), hypokalemia (3% versus 1%), hypoxia (3% versus 2%), and hypoventilation (2% versus <1%).
In a pharmacokinetic study of IONSYS™ conducted in 63 healthy volunteers (25 subjects older than 65 years), age did not significantly affect the extent of drug absorption. Literature suggests that the clearance of fentanyl may be reduced and the terminal half-life prolonged in the elderly (see PRECAUTIONS).
ADVERSE REACTIONS
In controlled and uncontrolled studies, the safety of IONSYS™ 40 mcg was evaluated in a total of 2114 patients with acute postoperative pain requiring opioid analgesia.
The most common adverse events (≥2%) in the placebo-controlled studies, regardless of relationship to study medication, are listed in Table 5.
|
* NOTE: Patients reported as having “Nausea and vomiting” are included in “Nausea” and “Vomiting” in Table 5. |
||
| Adverse Event | IONSYS™ (n=475) | Placebo (n=316) |
| Body as a Whole | ||
| Headache | 9% | 7 % |
| Fever | 9% | 10 % |
| Back Pain | 2% | 3 % |
| Cardiovascular | ||
| Hypotension | 2% | <1 % |
| Digestive* | ||
| Nausea | 39 % | 22 % |
| Vomiting | 12 % | 6 % |
| Hemic and Lymphatic | ||
| Anemia | 3 % | <1 % |
| Nervous system | ||
| Insomnia | 3 % | 5 % |
| Dizziness | 3 % | 1 % |
| Skin system | ||
| Application site reaction- Erythema | 14% | 2% |
| Pruritus | 6% | <1% |
| Urogenital | ||
| Urinary retention | 3% | <1% |
Adverse Events Reported in All Studies in Patients Treated With IONSYS™ (40 mcg/dose: n= 2114 including 3 Placebo-Controlled Trials and 4 Active Comparator Trials vs. IV PCA morphine)
The most common (>10%) adverse events reported regardless of relationship to IONSYS™ use were nausea, vomiting, application site reaction-erythema, fever, and headache. Other adverse events reported for IONSYS™ were:
(** indicates 1 to <10%, * indicates between 0.1 to <1%).
Body as a whole: abdominal pain**, back pain**, extremity pain**, pain**, injection site reaction*, chills*, internal postoperative bleeding*, chest pain*, infection*, injection site edema*, injection site pain*, immune system disorder*, abdomen enlarged*, asthenia*, neck pain*, abscess*, and hypothermia*, Cardiovascular System: hypotension**, tachycardia**, hypertension**, syncope*, postural hypotension*, pulmonary embolus*, atrial fibrillation*, bradycardia*, migraine*, myocardial infarct*, vasodilation*, hemorrhage*, deep thrombophlebitis*, bigeminy*, and arrhythmia*, Digestive System: constipation**, flatulence**, dyspepsia**, ileus**, gastrointestinal disorder*, dry mouth*, diarrhea*, and gastrointestinal hemorrhage*, Hemic and Lymphatic System: anemia**, and leukocytosis*, and Metabolic and Nutritional System: hypokalemia**, peripheral edema*, hypomagnesemia*, hypocalcemia*, hyponatremia*, hyperglycemia*, healing abnormal*, hypoglycemia*, hypophosphatemia*, edema*, and dehydration*, Musculoskeletal System: leg cramps* and myalgia*, Nervous System: dizziness**, insomnia**, anxiety**, hypertonia**, somnolence**, confusion*, paresthesia*, hypesthesia*, nervousness*, agitation*, abnormal dreams*, and tremor*, Respiratory System: hypoxia**, pharyngitis**, hypoventilation*, dyspnea*, apnea*, cough increased*, lung disorder*, asthma*, hiccup*, pneumonia*, atelectasis*, upper respiratory tract infection*, rhinitis*, sinusitis*, and hyperventilation*, Skin System: pruritus**, application site reaction (ASR)-itching**, ASR-vesicles**, ASR-edema**, ASR-other**, sweating**, wound site oozing/bleeding**, wound site inflammation/erythema*, rash*, ASR-dry and flaky*, ASR-papules/pustules*, vesiculobullous rash*, ASR-pain*, ASR-burning*, Special Senses: abnormal vision-blurred vision*, and ear pain*, Urogenital System: urinary retention**, urination impaired*, oliguria*, urogenital disorder*, hematuria*, urinary tract infection*, urinary urgency*, and dysuria*.
The level of current (62 microA/cm2) provided by IONSYS™ is generally imperceptible to the patient.
Scheduled observation of the skin approximately 24 hours after system removal was included in several studies. Some redness at the skin sites was observed in approximately 60% of patients at this observation. The skin findings included erythema, edema, and papules. The majority of these events were categorized as mild. Two patients were noted to have hyperpigmentation lasting 2-3 weeks at the application site. Three patients from another study noted a rectangular mark at the application site, which persisted for up to 3 months after study completion.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
IONSYS™ contains fentanyl, a Schedule II controlled substance.
Abuse
IONSYS™ contains a high concentration of a potent Schedule II controlled opioid agonist, fentanyl. Schedule II opioid substances, which include fentanyl, morphine, oxycodone, oxymorphone, hydromorphone, and methadone, have the highest potential for abuse. These drugs also have a risk of fatal overdose due to respiratory depression. Fentanyl can be abused and is subject to criminal diversion. The high drug content and concentrated formulation of fentanyl in IONSYS™ may be a particular target for abuse and diversion and may add to the risk of adverse outcomes from abuse. After the maximum dosage administration, a significant amount of fentanyl remains in the device.
Access to abusable drugs such as IONSYS™ presents a risk for abuse and diversion in the health care community. Implementation of effective accounting procedures in addition to routine procedures for handling controlled substances may minimize these risks.
Dependence
Tolerance and physical and psychological dependence may develop upon repeated administration of opioids. Iatrogenic addiction following opioid administration is relatively rare. Physicians should not let concerns of physical dependence deter them from using adequate amounts of opioids in the management of acute post-operative pain when such use is indicated.
OVERDOSAGE
Clinical Presentation
The manifestations of fentanyl overdosage are an extension of its pharmacologic actions, with the most serious effect being respiratory depression.
Treatment
For the management of respiratory depression, immediate countermeasures include removing IONSYS™ and discontinuing administration of any other opiates until the episode has resolved, as well as physically or verbally stimulating the patient. If needed, these actions can be followed by administration of a specific narcotic antagonist such as naloxone. Reversal of the narcotic effect may result in acute onset of pain and the release of catecholamines.
If the clinical situation warrants, ensure a patent airway is established and maintained, administer oxygen and assist or control respiration as indicated, and use an oropharyngeal airway or endotracheal tube if necessary. Adequate body temperature and fluid intake should be maintained.
If severe or persistent hypotension occurs, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy.
DOSAGE AND ADMINISTRATION
Patients should be titrated to comfort before initiating IONSYS™. Patients must have access to supplemental analgesia. IONSYS™ should be applied to intact, non-irritated and non-irradiated skin on the chest or upper outer arm.
IONSYS™ provides 40 mcg dose of fentanyl per activation on-demand. It is important to instruct patients how to operate IONSYS™ to self-administer doses of fentanyl as needed to manage their acute, short-term, postoperative pain. Only the patient should administer doses from IONSYS™. Each on-demand dose is delivered over a 10-minute period. To initiate administration of a fentanyl dose, the patient must press the button twice firmly within 3 seconds. An audible tone (beep) indicates the start of delivery of each dose; the red light remains on throughout the 10-minute dosing period.
Patients on chronic opioid therapy or with a history of opioid abuse may require higher analgesic doses in the post-operative period than are available from IONSYS™. Therefore, these patients should be evaluated frequently to ensure they are receiving adequate analgesia.
A maximum of six 40-mcg doses per hour can be administered by IONSYS™. The maximum amount of fentanyl that can be administered from a single IONSYS™ system over 24 hours is 3.2 mg (eighty 40-mcg doses). Each IONSYS™ system operates for 24 hours or until 80 doses have been administered, whichever occurs first. Up to three consecutive IONSYS™ systems may be used sequentially, each applied to a different skin site for a maximum of 72 hours of therapy for acute, short-term, postoperative pain.
IONSYS™ Testing Instructions for the Pharmacist or Other Health Care Professional (To be Performed Prior to Dispensing)
Each IONSYS™ should be tested before it is dispensed to a patient. The following functionality test should be performed by the pharmacist or pharmacy technician with IONSYS™ still in its sealed pouch:
- Hold the unopened foil pouch that contains the IONSYS™ system
- IONSYS™ button side is indicated on the pouch label
- Run a finger along the system until you feel the recessed button on one end
- Firmly press and release the button twice within 3 seconds (i.e., double-CLICK)
- Listen for a single audible tone (beep), confirming that the IONSYS™ system is functional and can be dispensed. If no tone is heard, the system is not functioning and should not be dispensed.
- The pharmacist should sign the front of pouch after performing a functionality test. The sticker on the back is intended for use by the registered Nurse (RN).
After a single audio tone is emitted based on this functionality test, a normally operating IONSYS™ system will also beep for 15 seconds after 4 minutes. This indicates that IONSYS™ is not in contact with the skin. Therefore, a functional system is confirmed by a single beep and/or 15 seconds of beeps after pressing the button. If a nurse or other health care professional performs the functionality test, and the system is applied to a patient within 4 minutes of having completed the test, the system will deliver the remainder of the 10-minute dose. For example, if the system is applied to the patient 3 minutes after the completion of the functionality test (i.e. when single beep is emitted), the system will deliver a 7-minute dose for this dose only. If a system is applied after the series of beeps is completed at 4 minutes, the system will deliver a 10-minute dose upon each activation.
If neither the single beep emitted upon double-pressing the dosing button nor the 15 second beeping after 4 minutes are heard, the system may be nonfunctional (see Troubleshooting.) For questions about IONSYS™, including product returns, please call Ortho-McNeil, Inc at 1-800-526-7736. Any nonfunctional system returned to the manufacturer should be returned in its intact package.
Do not open the pouch of a non-functional system and do not dispense it to a patient.
No drug is delivered from the system unless IONSYS™ is applied to the skin. Therefore, 80 doses and 24 hours of use are still available after the functionality test is performed.
Method of Application
IONSYS™ should be applied to intact, non-irritated, and non-irradiated skin on the chest or upper outer arm. IONSYS™ should not be placed on abnormal skin sites, such as scars, burns, tattoos, etc. Any excessive hair at the application site should be clipped (not shaved) before system application. Wipe the application site with a standard alcohol swab and allow the skin to dry completely before applying IONSYS™. Do not use any soaps, oils, lotions, or any other agents that might irritate the skin or alter its absorption characteristics.
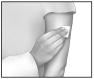
The sticker on the back of the pouch is intended for use by the registered nurse (RN). Fields on the sticker are provided for the nurse to write in the time and date IONSYS™ is applied to the patient. This sticker should be transferred from the pouch label to the IONSYS™ system that will be applied to the patient, in order to provide information to the next nurse on staff regarding when the 24-hour clock on IONSYS™ will expire.
To open the pouch containing IONSYS™, use scissors to cut along the dotted line of the pouch.


Remove and discard only the clear plastic liner covering the adhesive. Take care not to pull on the red tab while removing the clear plastic liner when preparing to apply IONSYS™ to the patient. The red tab is only to be used when separating the IONSYS™ system for disposal. (See disposal)
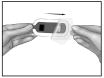
Press IONSYS™ firmly in place, with the sticky side down, on the skin for at least 15 seconds. Press with your fingers around the outer edges to be sure the system sticks to the skin.
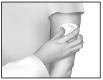
Occasionally, IONSYS™ may loosen; if this occurs a non-allergenic tape may be used to be sure all of the system's edges make complete contact with the skin. Take care not to tape over the button or the red light.
Each IONSYS™ may be used for 24 hours from completion of the first on-demand dose or until 80 doses have been administered, whichever comes first. After the 24 hours have elapsed, or 80 doses have been delivered, IONSYS™ is deactivated and cannot deliver any additional doses i.e. if the patient tries to initiate a dose, the system will not beep and the red LED will not light up continuously. At this time the red LED will continue to flash indicating the approximate number of doses delivered to the patient up until the time the system was deactivated. The LED will continue to flash until the battery in the system is depleted. If additional opioid analgesia is required, a new system should be applied to a different skin site after removal and disposal of the previous system.
Dose Delivery
A recessed button and red light are located on the top housing of IONSYS™. To initiate a fentanyl dose, the patient should press the button twice within 3 seconds. An audible tone (beep) indicates the start of delivery of each dose; the red light remains on throughout the 10-minute dosing interval. The next dose cannot begin until the previous 10-minute delivery cycle is complete. Pressing the button during delivery of a dose will not result in additional drug being administered. The red light turns off after each 10-minute dose has been delivered.
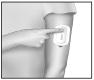
A healthcare professional should observe the first dose administered to ensure that the patient understands how to operate IONSYS™ and that the system is working properly.
Determining Approximate Number of Doses Delivered
Between doses, the red light will flash in one-second pulses to indicate the approximate number of doses that have been administered up to the present time. Each one-second flash of light indicates administration of up to five doses. Thus, a single one-second flash of light represents 1 to 5 doses; two flashes represent 6 to 10 doses; three flashes represent 11 to 15 doses; and so on up to 16 flashes which represent 76 to 80 doses delivered. The system may also be queried during delivery of an on-demand dose, by a single press of the button. The red light will flash as outlined above to indicate the approximate number of on-demand doses that have been delivered up to the time of the query. This query will not influence dose delivery.
| No. of light flashes | No. of fentanyl doses delivered | Range (mcg) of fentanyl delivered |
| 1 | 1-5 | 40-200 |
| 2 | 6-10 | 240-400 |
| 3 | 11-15 | 440-600 |
| 4 | 16-20 | 640-800 |
| 5 | 21-25 | 840-1,000 |
| 6 | 26-30 | 1040-1200 |
| 7 | 31-35 | 1240-1400 |
| 8 | 36-40 | 1440-1600 |
| 9 | 41-45 | 1640-1800 |
| 10 | 46-50 | 1840-2000 |
| 11 | 51-55 | 2040-2200 |
| 12 | 56-60 | 2240-2400 |
| 13 | 61-65 | 2440-2600 |
| 14 | 66-70 | 2640-2800 |
| 15 | 71-75 | 2840-3000 |
| 16 | 76-80 | 3040-3200 |
Removal
IONSYS™ may be removed at any time. However, once a system has been removed, the same system should not be reapplied.

At the end of 24 hours of use, or after 80 doses have been delivered, the IONSYS™ system will deactivate and should be removed from the patient's skin. Using gloves, remove IONSYS™ by gently lifting the red tab and loosening the system from the skin application site. Ensure both hydrogels remain with the removed IONSYS™ system. If the hydrogel becomes separated from the IONSYS™ system during removal, use gloves or tweezers to remove the hydrogel from the skin and properly dispose of in accordance with state and federal regulations for controlled substances. If the patient requires additional pain relief, a new IONSYS™ system should be applied. In this case, IONSYS™ should be applied to a new skin site on the upper outer arm or chest.
Take care not to touch the exposed hydrogel compartments or the adhesive. If a hydrogel drug reservoir is touched accidentally, rinse the area thoroughly with water (do not use soap).
Disposal
Contact with the hydrogels contained in IONSYS™ can be harmful to humans and animals. Medical staff should handle the removed system carefully and only by the sides and top housing. Disposal should be in accordance with state and federal regulations for controlled substances. The used bottom housing of IONSYS™ contains a significant amount of fentanyl that could be harmful or fatal if ingested and which could be diverted for abuse.
To dispose of a used IONSYS™:
- Using gloves, pull the red tab to separate the bottom housing from the top housing.

- Fold the bottom hydrogel-containing housing in half with the sticky side facing in.

- Dispose of the folded over bottom housing, containing the residual fentanyl, by flushing this piece down the toilet. This step should be witnessed by a second health care provider. The used bottom housing of IONSYS™ contains fentanyl that could be harmful or fatal if ingested.
- Dispose of top housing, containing electronics, according to hospital procedures for battery-containing waste.
- If the hydrogel accidentally contacts the skin, rinse the affected area thoroughly with water (do not use soap). Contact with the fentanyl hydrogel contained in IONSYS™ can be harmful to humans and animals. Oral ingestion or contact of the hydrogels with mucous membranes may cause serious illness or death. Do not allow hydrogel to touch mouth.
Troubleshooting
IONSYS™ is designed to deliver each on-demand dose of fentanyl over approximately 10 minutes. Please refer to the Normal System Feedback table below which illustrates normal audio and visual feedback from the IONSYS™ system, both during the in-pouch functionality testing that is performed before the system is dispensed to a patient, and during patient use.
| Mode | Audio Feedback | Visual Feedback |
| IONSYS™ functionality testing (performed prior to dispensing to patient): | ||
| 1. With the IONSYS™ system in its unopened pouch, run a finger along the system until the recessed dosing button is felt (pouch label indicates the side on which the button will be located) 2. Firmly press and release the button twice within 3 seconds | Immediately after step 2 is performed, the system will emit a single beep, indicating a functional system. Approximately four minutes later, the system will also emit a series of short beeps (15 seconds duration). This is normal and does not affect the functionality or life of the product once applied to the patient. | None (system is in opaque foil pouch). Note that if this test were performed with IONSYS™ outside of the pouch and off the patient's body, the red LED would illuminate for 4 minutes and then turn off at the time the 15 seconds beeping starts. |
| During and After Patient Use | ||
| Initiate administration of a dose by pressing the button twice firmly within 3 seconds. | A single audio tone (beep) indicates the start of delivery of each dose. | The red light remains solidly illuminated throughout each 10- minute dosing period. |
| Advise Patient that when system is flashing, he or she can self-administer another dose of fentanyl from the system as needed for pain relief | None. | At any time within 24 hours of initiation of first dose from system, and < 80 doses have been delivered, system displays flashing red light to give an approximation of the fentanyl doses that have been administered. Each series of flashes, followed by a 2 second pause, will repeat until the patient administers the next dose. Each flash of light represents administration of 1-5 fentanyl doses (40 mcg each) |
| System deactivates after 24 hours have elapsed from time of first dose administered, or if all 80 doses have been used. You may determine the number of doses administered by counting the number of flashes during one series. Each pulse of light represents the administration of 1-5 fentanyl doses (40 mcg each) | None. | Displays flashing red light after 24 hours have elapsed from time of initiation of the first dose from system, OR all 80 doses have been delivered. System is deactivated and a new system must be applied to a new skin site if needed |
If a system does not appear to function immediately, wait 4 minutes and firmly press and release the button twice within 3 seconds (i.e., double-press). A single audio beep will be emitted immediately, confirming that the IONSYS™ system is functional. If a system is not functioning properly, instruct the patient to call a staff member to observe the next time he/she initiates a dose. Then monitor both the illumination of the red light and any error messages (beeps) that may be provided by the system. Refer to the Error Messages chart below for possible problems and appropriate course of action.
Error messages provide information about problems that may occur during operation of IONSYS™. These messages are in the form of alarms consisting of a series of audible signals or “beeps” as follows:
| Error Message/Feedback | Probable Cause | Action |
| Emits a 15 second series of beeps (this short series of beeps repeats 8 times then stops). | Decreased fentanyl delivery. Could be caused by poor contact between the system and the skin or due to high skin resistance. | Ensure good contact is made between the gels and the skin. Attempt to restart system (up to 3 times). If required, a new system should be applied to a new skin site. |
| Emits a continuous series of beeps that repeat until the battery is depleted (i.e. beeps cannot be shut off). | IONSYS™ placed on compromised skin site, increased output current detected, or low battery voltage. | Remove system from patient. System cannot be restarted. Apply new system to a new site. |
| No single beep emitted, and no solid red light illuminated upon dose activation. Red light is not flashing. | Depleted battery or non-functional system. | Remove system, and if required, a new system should be applied to a new skin site. |
Discontinuation of IONSYS™
To convert patients to another opioid or other analgesic, remove IONSYS™ and titrate the dose of the new analgesic, based upon the patient's report of pain, until adequate analgesia has been obtained, using caution due to the fact that serum fentanyl concentration will decrease slowly following removal of the system (See Figure 1b; WARNINGS). Following cessation of IONSYS™ treatment in clinical trials, patients were typically administered oral analgesics; a small percentage of patients received parenteral opioids.
HOW SUPPLIED
IONSYS™ is provided as a disposable system providing up to 80 doses (40 mcg each) of fentanyl. Each IONSYS™ is individually packaged in a child-resistant foil pouch. There are five packages per carton.
NDC # 0062-7900-01 (single IONSYS™ system in foil pouch)
NDC # 0062-7900-05 (carton of 5 IONSYS™ systems)
Storage and Handling
Accidental ingestion of the fentanyl hydrogel or contact of the hydrogel with mucous membranes could lead to the absorption of a potentially fatal dose of fentanyl. Therefore, the hydrogels should not come into contact with fingers or mouth.
Avoid direct contact with the fentanyl hydrogel and the adhesive surface during system application and removal. If the hydrogel from the drug reservoir accidentally contacts the skin, the affected area should be rinsed thoroughly with water. Do not use soap, alcohol, or other solvents to remove the hydrogel because they may enhance the drug's ability to penetrate the skin.
IONSYS™ should be stored at 25°C (77°F); excursions permitted to 15 – 30°C (59-86°F). Apply to the skin immediately after removal from the individually sealed package. Do not use if the foil pouch has been broken. For transdermal use only.
For questions about IONSYS™, including product returns please call Ortho-McNeil, Inc. Medical Communications Contact Center at 1-800-526-7736.
Rx only
Marketed by:
Ortho-McNeil, Inc.
Raritan, NJ 08869
Manufactured by:
ALZA Corporation
Mountain View, CA 94043
An ALZA E-TRANS® Technology Product
Revised August 2006 (0016720-2)
PATIENT INFORMATION
Patient Bedside Information Sheet
IONSYS™ (eye-AHN-sis)
(fentanyl iontophoretic transdermal system)
Patient-activated CII
40 mcg per activation
IMPORTANT: Never leave the hospital with an IONSYS™ system on your skin. IONSYS™ is not for home use. IONSYS™ can cause life-threatening breathing problems or death if it is used the wrong way.
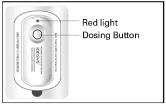
What is IONSYS™?
IONSYS™:
- contains the prescription medicine, fentanyl. Fentanyl is a very strong narcotic pain medicine (opioid).
- is only for hospitalized adults with short-term, moderate to severe pain after surgery
- is a patient-controlled medicine device (system) that sticks to the skin
IONSYS™ is not:
- for use at home
- for patients who cannot understand or use IONSYS™ without help
-
for patients who are allergic to:
- fentanyl
- Cepacol® (cetylpiridinum chloride)
Your nurse or doctor:
- will tell you about IONSYS™ and teach you how to use it before your surgery
- will put an IONSYS™ system on your skin (on your upper outer arm or chest) after your surgery
- will control pain from your surgery with other pain medicines until you are awake enough to use IONSYS™
- will monitor you for side effects from IONSYS™. IONSYS™ can cause life-threatening breathing problems because it contains a very strong narcotic pain medicine.
- will replace your IONSYS™ system as needed
- will remove your IONSYS™ system before you leave the hospital

Using IONSYS™:
- You can push the IONSYS™ dosing button when needed to control your pain or just before you do an activity that may increase your pain such as physical therapy or getting out of bed.
- To get a dose of pain medicine from IONSYS™, double-click the dosing button (twice within 3 seconds).
- When you push the dosing button you will hear a single beep and the red light above the dosing button will light up. This red light will remain lit for the 10 minutes it takes to deliver a dose of fentanyl.
- You cannot give yourself another dose of medicine from IONSYS™ while the red light is lit.
- When IONSYS™ is finished delivering a dose, the red light will start flashing. This means you can give yourself more pain medicine, if needed, by double clicking the dosing button. The flashes also tell your doctor and nurse about how many doses of medicine from IONSYS™ that you have taken.
- Let your doctor or nurse know if you have problems using IONSYS™. They will check your IONSYS™ system to make sure it is working.
Never:
- Never let anyone else press the IONSYS™ dosing button for you.
- Never touch the sticky side of IONSYS™ if it falls off of your skin. Let your nurse or doctor know right away if an IONSYS™ system has come off of your skin. Wash your hands right away if you accidentally touch the sticky side of IONSYS™ and let your doctor or nurse know right away.
- Never remove or replace an IONSYS™ system yourself.
- Never leave the hospital with an IONSYS™ system on your skin. Make sure your nurse or doctor removes your IONSYS™ system before you leave the hospital
| IONSYS (fentanyl hydrochloride) | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
Revised: 07/2007Ortho-McNeil, Inc.