PROPINE
-
dipivefrin hydrochloride solution/ drops
Allergan, Inc.
----------
DESCRIPTION
PROPINE® contains dipivefrin hydrochloride in a sterile, isotonic solution. Dipivefrin HCI is a white, crystalline powder, freely soluble in water with an osmolality of approximately 250 - 330 mOsmol/kg.
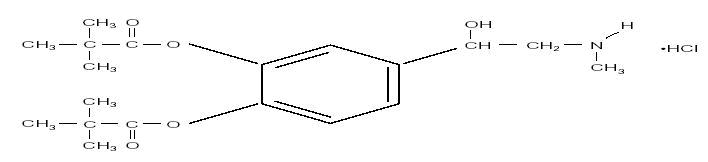
Empirical Formula:
C19H29O5N•HCI
Chemical Name: (±) -3,4-Dihydroxy–α– [(methylamino) methyl] benzylalcohol 3,4-dipivalate hydrochloride.
STRUCTURAL FORMULA:
Contains: Active: dipivefrin HCI 0.1%
Preservative: benzalkonium chloride
Inactives: edetate disodium; purified water; sodium chloride; and hydrochloric acid to adjust pH. The pH during its shelf life ranges from 2.5 - 3.5.
CLINICAL PHARMACOLOGY
PROPINE® (dipivefrin HCI ophthalmic solution, USP) is a member of a class of drugs known as prodrugs. Prodrugs are usually not active in themselves and require biotransformation to the parent compound before therapeutic activity is seen. These modifications are undertaken to enhance absorption, decrease side effects and enhance stability and comfort, thus making the parent compound a more useful drug. Enhanced absorption makes the prodrug a more efficient delivery system for the parent drug because less drug will be needed to produce the desired therapeutic response.
PROPINE® ophthalmic solution is a prodrug of epinephrine formed by the diesterification of epinephrine and pivalic acid. The addition of pivaloyl groups to the epinephrine molecule enhances its lipophilic character and as a consequence, its penetration into the anterior chamber.
PROPINE® is converted to epinephrine inside the human eye by enzyme hydrolysis. The liberated epinephrine, an adrenergic agonist, appears to exert its action by decreasing aqueous production and by enhancing outflow facility. The PROPINE® prodrug delivery system is a more efficient way of delivering the therapeutic effects of epinephrine, with fewer side effects than are associated with conventional epinephrine therapy.
The onset of action with one drop of PROPINE® occurs about 30 minutes after treatment, with maximum effect seen at about one hour.
Using a prodrug means that less drug is needed for therapeutic effect since absorption is enhanced with the prodrug. PROPINE® ophthalmic solution at 0.1% dipivefrin was judged less irritating than a 1% solution of epinephrine hydrochloride or bitartrate. In addition, only 8 of 455 patients (1.8%) treated with PROPINE® reported discomfort due to photophobia, glare or light sensitivity.
INDICATIONS
PROPINE® (dipivefrin HCI ophthalmic solution, USP) is indicated as initial therapy for the control of intraocular pressure in chronic open-angle glaucoma. Patients responding inadequately to other antiglaucoma therapy may respond to addition of PROPINE®.
In controlled and open-label studies of glaucoma, PROPINE® ophthalmic solution demonstrated a statistically significant intraocular pressure-lowering effect. Patients using PROPINE® twice daily in studies with mean durations of 76-146 days experienced mean pressure reductions ranging from 20-24%.
Therapeutic response to PROPINE® ophthalmic solution twice daily is somewhat less than 2% epinephrine twice daily. Controlled studies showed statistically significant differences in lowering of intraocular pressure between PROPINE® and 2% epinephrine. In controlled studies in patients with a history of epinephrine intolerance, only 3% of patients treated with PROPINE® ophthalmic solution exhibited intolerance, while 55% of those treated with epinephrine again developed intolerance.
Therapeutic response to PROPINE® twice daily therapy is comparable to 2% pilocarpine 4 times daily. In controlled clinical studies comparing PROPINE® ophthalmic solution and 2% pilocarpine, there were no statistically significant differences in the maintenance of IOP levels for the two medications. PROPINE® does not produce miosis or accommodative spasm which cholinergic agents are known to produce. Night blindness often associated with miotic agents is not present with PROPINE® therapy. Patients with cataracts avoid the inability to see around lenticular opacities caused by constricted pupil.
CONTRAINDICATIONS
PROPINE® should not be used in patients with narrow angles since any dilation of the pupil may predispose the patient to an attack of angle-closure glaucoma. This product is contraindicated in patients who are hypersensitive to any of its components.
PRECAUTIONS
Aphakic Patients.
Macular edema has been shown to occur in up to 30% of aphakic patients treated with epinephrine. Discontinuation of epinephrine generally results in reversal of the maculopathy.
Pregnancy:
Pregnancy Category B. Reproduction studies have been performed in rats and rabbits at daily oral doses up to 10 mg/kg body weight (5 mg/kg in teratogenicity studies), and have revealed no evidence of impaired fertility or harm to the fetus due to dipivefrin HCI. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PROPINE® ophthalmic solution is administered to a nursing woman.
ADVERSE REACTIONS
Cardiovascular Effects. Tachycardia, arrhythmias and hypertension have been reported with ocular administration of epinephrine.
Local Effects.The most frequent side effects reported with PROPINE® alone were injection in 6.5% of patients and burning and stinging in 6%. Follicular conjunctivitis, eye pain, mydriasis, blurry vision, eye pruritus, headache and allergic reaction to PROPINE® ophthalmic solution have been reported. Epinephrine therapy can lead to adrenochrome deposits in the conjunctiva and cornea.
DOSAGE AND ADMINISTRATION
Replacement with PROPINE® ophthalmic solution. When patients are being transferred to PROPINE® from antiglaucoma agents other than epinephrine, on the first day continue the previous medication and add one drop of PROPINE® ophthalmic solution in each eye every 12 hours. On the following day, discontinue the previously used antiglaucoma agent and continue with PROPINE®.
In transferring patients from conventional epinephrine therapy to PROPINE® ophthalmic solution, simply discontinue the epinephrine medication and institute the PROPINE® regimen.
HOW SUPPLIED
PROPINE® (dipivefrin HCI ophthalmic solution, USP) 0.1%, is supplied sterile in opaque white low density polyethylene ophthalmic dispenser bottles and tips with purple polystyrene caps as follows:
| 10 mL in 10 mL bottle | - NDC 0023-9208-10 | |
| 15 mL in 15 mL bottle | - NDC 0023-9208-15 |
Note: Store in a tight, light-resistant container at 15° to 25°C (59° to 77°F).
Rx Only
Revised December 2005
© 2006 Allergan, Inc.
Irvine, CA 92612, U.S.A.
® marks owned by Allergan, Inc.
7592X
71738US11T
®ALLERGAN
NDC 0023-9208-10 Rx Only
PROPINE®
(dipivefrin HCl ophthalmic
solution, USP) 0.1%
10 mL sterile
®ALLERGAN
NDC 0023-9208-10
Rx Only
PROPINE®
(dipivefrin HCl
ophthalmic
solution,
USP) 0.1%
sterile
10 mL
| PROPINE
dipivefrin hydrochloride solution/ drops |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA018239 | 09/04/2001 | 01/04/2010 |
| Labeler - Allergan, Inc. (144796497) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Allergan, Inc. | 362898611 | MANUFACTURE | |
Revised: 06/2011 Allergan, Inc.