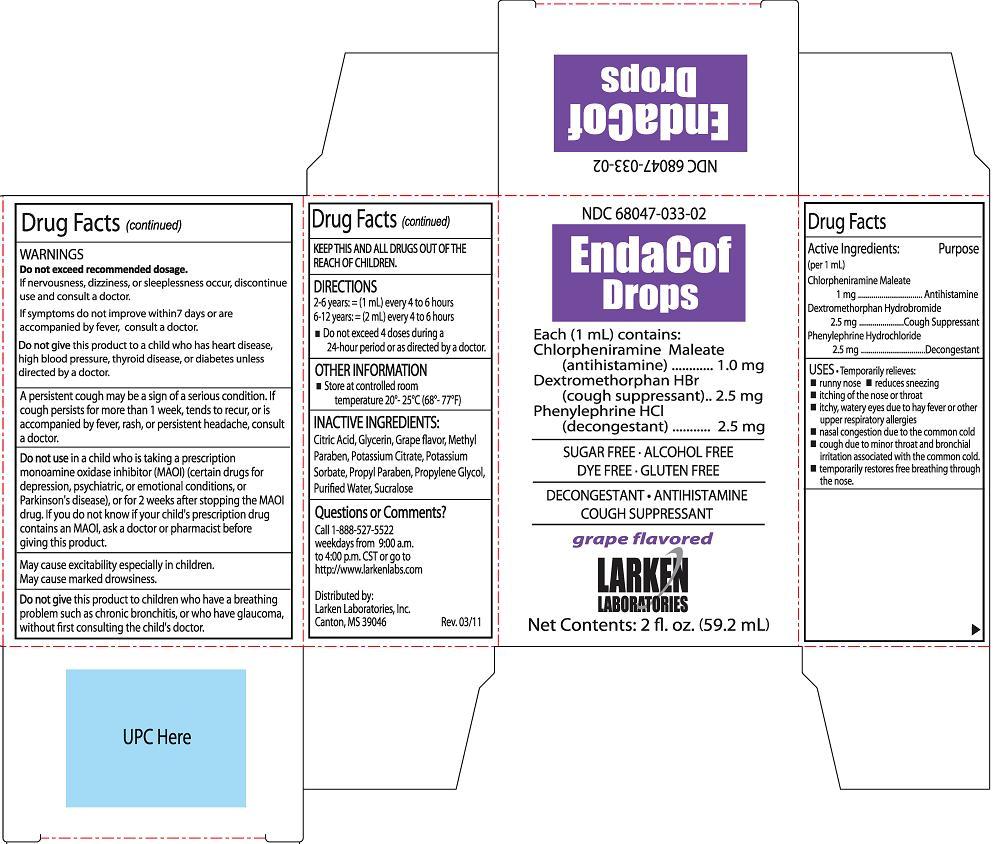
ENDACOF DROPS
-
chlorpheniramine maleate,
dextromethorphan hydrobromide and
phenylephrine hydrochloride liquid
Larken Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
(per 1 mL)
Chlorpheniramine Maleate 1 mg
Dextromethorphan Hydrobromide 2.5mg
Phenylephrine Hydrochloride 2.5mg
Purpose
Chlorpheniramine Maleate Antihistamine
Dextromethorphan HBr Cough Suppressant
Phenylephrine HCl Decongestant
Uses
Temporarily relieves:
- runny nose
- reduces sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever or other respiratory allergies
- nasal congestion due to the common cold
- cough due to minor throat and bronchial irritation associated with the common cold
- temporarily restores free breathing through the nose
Stop use and ask a doctor
- If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor.
- If symptoms do not improve within 7 days or are accompanied by fever, consult a doctor.
Do not use
- this product for a child who has heart disease, high blood pressure, thyroid disease, or diabetes unless directed by a doctor.
- this product for persistent or chronic cough such as occurs with asthma or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
- A persistent cough may be a sign of a serious condition.
- If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use
- for children who have a breathing problem such as chronic bronchitis, or who have glaucoma, without first consulting the child's doctor.
Directions
2-6 years: = (1 mL) every 4 to 6 hours
6-12 years: = (2 mL) every 4 to 6 hours
- Do not exceed 4 doses during a 24-hour period or as directed by a Doctor
Inactive Ingredients
Citric Acid, Glycerin, Grape flavor, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sucralose
Questions or Comments
Call 1-888-527-5522 weekdays from 9:00 am to 4:00 pm CST or go to http://www.larkenlabs.com.
| ENDACOF DROPS
chlorpheniramine maleate, dextromethorphan hydrobromide and phenylephrine hydrochloride liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 05/16/2011 | |
| Labeler - Larken Laboratories, Inc. (791043719) |
| Registrant - Larken Laboratories, Inc. (791043719) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| T.G. United | 830980947 | MANUFACTURE | |
Revised: 05/2011 Larken Laboratories, Inc.