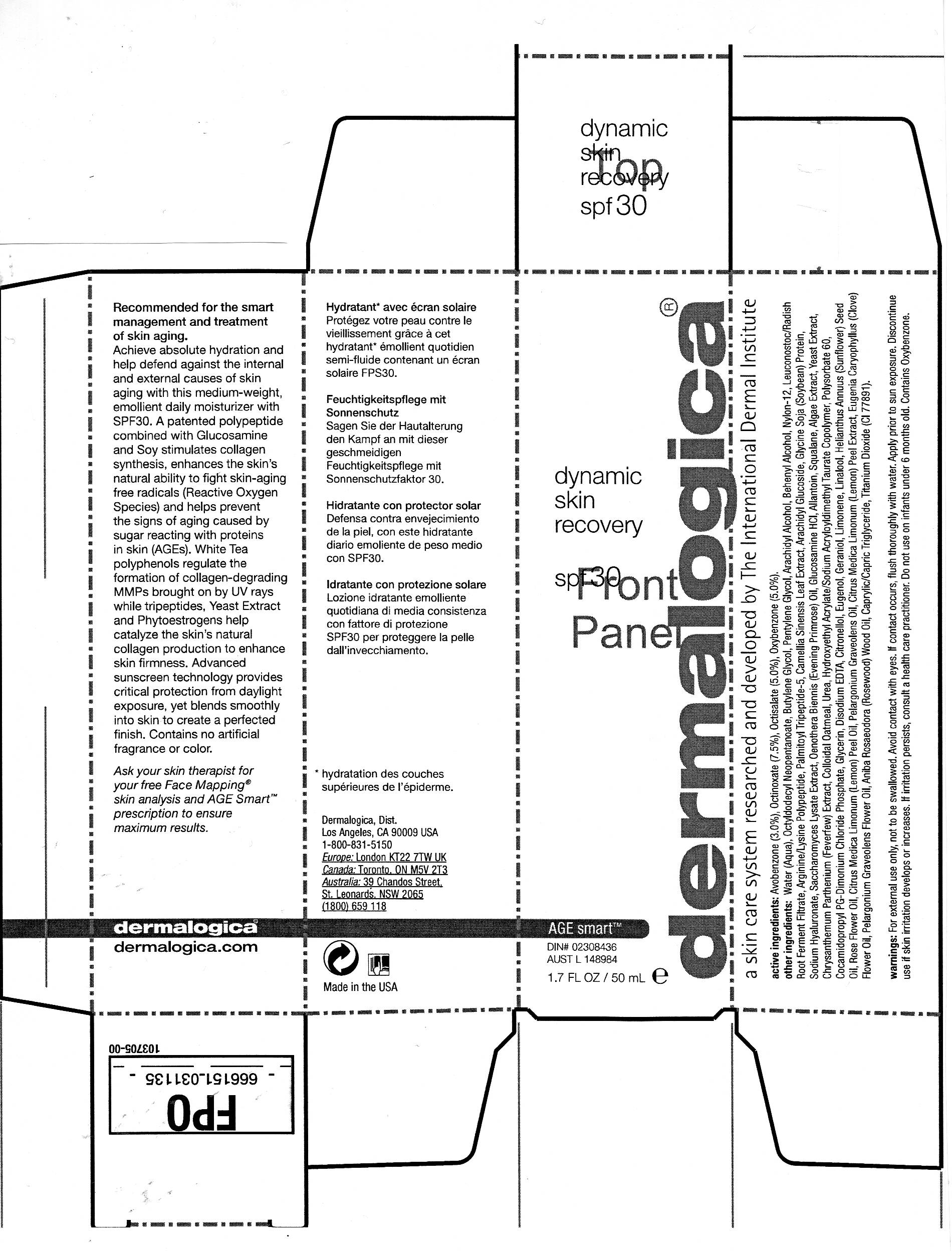
DYNAMIC SKIN RECOVERY SPF30
-
avobenzone,
octinoxate,
oxybenzone and
octisalate lotion
PureTek Corporation
----------
active ingredients:
Avobenzone (3.0%), Octinoxate (7.5%), Octisalate (5.0%), Oxybenzone (5.0%).
other ingredients:
Water (Aqua), Octyldodecyl Neopentanoate, Butylene Glycol, Pentylene Glycol, Arachidyl Alcohol, Behenyl Alcohol, Nylon-12, Leuconostoc/Radish Root Ferment Filtrate, Arginine/Lysine Polypeptide, Pamlitoyl Tripeptide-5, Camellia Sinensis Leaf Extract, Arachidyl Glycoside, Glycine Soja (Soybean) Protein, Sodium Hyaluronate, Saccharomyces Lysate Extract, Oenothera Biennis (Evening Primrose) Oil, Glucosamine HCl, Allantoin, Squalane, Algae Extract, Yeast Extract, Chrysanthemim Parthenium (Feverfew) Extract, Colloidal Oatmeal, Urea, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polysorbate 60, Cocamidopropyl PG-Dimonium Chloride Phosphate, Glycerin, Disodium EDTA, Citronellol, Eugenol, Geraniol, Limonene, Linalool, Helianthus Annuus (Sunflower) Seed Oil, Rose Flower Oil, Citrus Medica Limonum (Lemon) Peel Oil, Pelargonium Graveolens Oil, Citrus Medica Limonum (Lemon) Peel Extract, Eugenia Caryophyllus (Clove) Flower Oil, Pelargonium Groveolens Flower Oil, Aniba Rosaeodora (Rosewood) Wood Oil, Caprylic/Capric Triglyceride,Titanium Dioxide (Cl 77891).
directions:
Apply to face and neck, preferably 30 minutes prior to daylight exposure.
warnings:
For external use only, not to be swallowed. Avoid contact with eyes. If contact occurs, flush thoroughly with water. Apply prior to sun exposure. Discontinue use if skin irritation develops or increases. If irritation persists, consult a health care practitioner. Do not use on infants under 6 months old. Contains Oxybenzone.
Label
DYNAMIC SKIN RECOVERY SPF30
avobenzone, octinoxate, octisalate, oxybenzone
lotion |
|
|
|
|
|
|
|
|
|
|
Revised: 05/2011PureTek Corporation