ESTOMAROL
-
aluminum hydroxide,
bismuth subcarbonate,
calcium carbonate,
magnesium carbonate and
sodium bicarbonate powder
Laboratorios Imperiales, S.A. de C.V.
----------
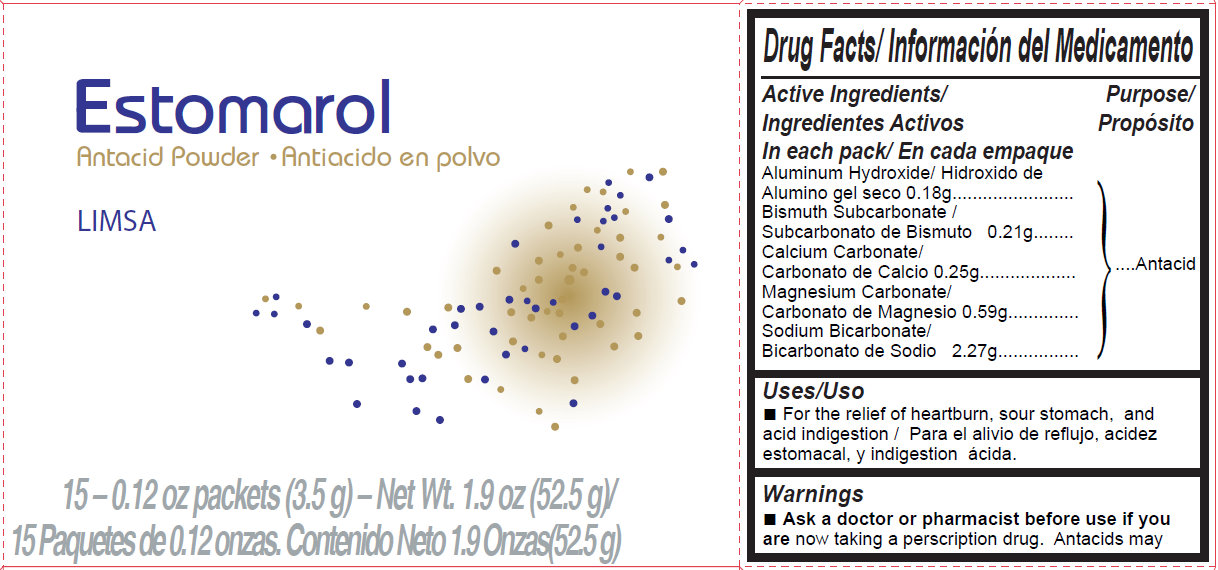
Estomarol Antacid PowderDrug Facts
Active Ingredients
In each pack
Aluminum Hydroxide 0.18g
Bismuth Subcarbonate 0.21g
Calcium Carbonate 0.25g
Magnesium Carbonate 0.59g
Sodium Bicarbonate 2.27g
Purpose
Antacid
Uses
- For the relief of heartburn, sour stomach, and acid indigestion
Directions
Add packet (3.5g) to a cup of water after each meal taken, maximum of 6 doses in 24 hours or as directed by a physician.
Other information
In each pack:
Calcium 95mg, Magnesium 145mg, and Sodium 605mg.
Inactive Ingredients
Colloidal silicon dioxide, glycerine anyhydrous, peppermint oil
Warnings
- Ask a doctor or pharmacist before use if you are now taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- Do not take more than 6 doses in 24-hour period, or use more than 2 weeks, except under the advice and supervision of a physician.
- Do not use this product except under the advice and supervision of a physician if you have kidney disease or are on a sodium restricted diet.
- Do not take this product if you are presently taking a prescription antibiotic drug containing any form of tetracycline.
- Do not use if you are pregnant or lactating.
- Use caution in patients who are taking anticoagulants or who are diabetic.
- Do not administer to adolescents who have or have recently had chicken pox
- This medication can cause constipation and in large doses can cause diarrhea, dark urine and dark bowel movements.
- Do not give to children under 6
Keep out of reach of children.
Estomarol
Antacid Powder
LIMSA
15-0.12 oz packets (3.5g) - Net Wt. 1.9 oz (52.5 g)




| ESTOMAROL
aluminum hydroxide, bismuth subcarbonate, calcium carbonate, magnesium carbonate, sodium bicarbonate powder |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part331 | 05/11/2011 | |
| Labeler - Laboratorios Imperiales, S.A. de C.V. (812643138) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Laboratorios Imperiales, S.A. de C.V. | 812643138 | manufacture | |