KUVAN
-
sapropterin dihydrochloride tablet
BioMarin Pharmaceutical Inc.
----------
|
|||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
KuvanTM (sapropterin dihydrochloride) Tablets is indicated to reduce blood phenylalanine (Phe) levels in patients with hyperphenylalaninemia (HPA) due to tetrahydrobiopterin- (BH4-) responsive Phenylketonuria (PKU). Kuvan is to be used in conjunction with a Phe-restricted diet.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
The recommended starting dose of Kuvan is 10 mg/kg/day taken once daily.
Response to therapy is determined by change in blood Phe following treatment with Kuvan at 10 mg/kg/day for a period of up to 1 month. Blood Phe levels should be checked after 1 week of Kuvan treatment and periodically for up to a month. If blood Phe does not decrease from baseline at 10 mg/kg/day, the dose may be increased to 20 mg/kg/day. Patients whose blood Phe does not decrease after 1 month of treatment at 20 mg/kg/day are non-responders, and treatment with Kuvan should be discontinued in these patients.
Once responsiveness to Kuvan has been established, the dosage may be adjusted within the range of 5 to 20 mg/kg/day according to response to therapy. Doses of Kuvan above 20 mg/kg/day have not been evaluated in clinical trials.
2.2 Administration
KuvanTM (sapropterin dihydrochloride) Tablets should be administered orally with food to increase absorption, preferably at the same time each day. Kuvan Tablets should be dissolved in 4 to 8oz. (120 to 240 mL) of water or apple juice and taken within 15 minutes of dissolution. It may take a few minutes for the tablets to dissolve. To make the tablets dissolve faster, stir or crush them. The tablets may not dissolve completely. Patients may see small pieces floating on top of the water or apple juice. This is normal and safe for patients to swallow. If after drinking the medicine patients still see pieces of the tablet, they can add more water or apple juice to make sure that they take all of the medicine. A missed dose should be taken as soon as possible, but 2 doses should not be taken on the same day.
3 DOSAGE FORMS AND STRENGTHS
Kuvan TM (sapropterin dihydrochloride) Tablets are unscored, uncoated, immediate-release tablets for oral use. Each tablet contains 100 mg of sapropterin dihydrochloride (equivalent to 76.8 mg of sapropterin base). Tablets are round, off-white to light yellow, mottled, and debossed with “177”.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Monitor Blood Phe Levels During Treatment
Treatment with Kuvan should be directed by physicians knowledgeable in the management of PKU. Prolonged elevations in blood Phe levels in patients with PKU can result in severe neurologic damage, including severe mental retardation, microcephaly, delayed speech, seizures, and behavioral abnormalities. This may occur even if patients are taking Kuvan but not adequately controlling their blood Phe levels within recommended target range. Long-term studies of neurocognitive outcomes with Kuvan treatment have not been conducted. Conversely, prolonged levels of blood Phe that are too low have been associated with catabolism and protein breakdown. Active management of dietary Phe intake while taking Kuvan is required to ensure adequate Phe control and nutritional balance.
5.2 Identify Non-Responders to Kuvan Treatment
Not all patients with PKU respond to treatment with Kuvan. In clinical trials, approximately 20% to 56% of PKU patients responded to treatment with Kuvan [seeClinical Studies (14.1)]. Response to treatment cannot be pre-determined by laboratory testing (e.g., genetic testing), and can only be determined by a therapeutic trial of Kuvan [seeDosage and Administration (2.1)].
5.3 Treat All Patients With a Phe-restricted Diet
Patients with PKU who are being treated with Kuvan should also be treated with a Phe-restricted diet. The initiation of Kuvan therapy does not eliminate the need for appropriate monitoring by trained professionals to assure that blood Phe control is maintained in the context of ongoing dietary management.
5.4 Use With Caution in Patients With Hepatic Impairment
Patients with liver impairment have not been evaluated in clinical trials with Kuvan. Patients who have liver impairment should be carefully monitored when receiving Kuvan because hepatic damage has been associated with impaired Phe metabolism.
5.5 Monitor for Allergic Reactions
Patients who have a known severe allergy to any of the components of Kuvan should not take Kuvan. In clinical trials conducted with Kuvan, no severe allergic reactions were observed. The risks and benefits of continued treatment with Kuvan in patients with mild to moderate allergic reactions (such as rash) should be considered.
5.6 Use With Caution When Co-administering Kuvan and Medications Known to Inhibit Folate Metabolism
Drugs known to affect folate metabolism (e.g., methotrexate) and their derivatives should be used with caution while taking Kuvan because these drugs can decrease BH4 levels by inhibiting the enzyme dihydropteridine reductase (DHPR).
5.7 Use With Caution When Co-administering Kuvan and Drugs Known to Affect Nitric Oxide-Mediated Vasorelaxation
Caution should be used with the administration of Kuvan to patients who are receiving drugs that affect nitric oxide-mediated vasorelaxation (e.g., PDE-5 inhibitors such as sildenafil, vardenafil, or tadalafil), because both sapropterin dihydrochloride and PDE-5 inhibitors may induce vasorelaxation. The additive effect of sapropterin and PDE-5 inhibitor co-administration could lead to a reduction in blood pressure; however, the combined use of these medications has not been evaluated in humans. In animal studies, orally administered Kuvan in combination with a PDE-5 inhibitor had no effect on blood pressure.
5.8 Use With Caution When Co-administering Kuvan and Levodopa
Caution should be used with the administration of Kuvan to patients who are receiving levodopa. In a 10-year post-marketing safety surveillance program for a non-PKU indication using another formulation of the same active ingredient (sapropterin), 3 patients with underlying neurologic disorders experienced convulsions, exacerbation of convulsions, over-stimulation, or irritability during co-administration of levodopa and sapropterin.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience in PKU
In clinical trials, Kuvan has been administered to 579 patients with PKU in doses ranging from 5 to 20 mg/kg/day for lengths of treatment ranging from 1 to 30 weeks. Patients were aged 4 to 49 years old. The patient population was nearly evenly distributed in gender, and approximately 95% of patients were Caucasian.
The most serious adverse reactions during Kuvan administration (regardless of relationship to treatment) were gastritis, spinal cord injury, streptococcal infection, testicular carcinoma, and urinary tract infection. Mild to moderate neutropenia was noted during Kuvan administration in 24 of 579 patients (4%). The most common (≥4% of patients treated with Kuvan) across all studies (n=579) were headache, diarrhea, abdominal pain, upper respiratory tract infection, pharyngolaryngeal pain, vomiting, and nausea.
The data described below reflect exposure of 74 patients with PKU to Kuvan at doses of 10 to 20 mg/kg/day for 6 to 10 weeks in 2 double-blind, placebo-controlled clinical trials. The overall incidence of adverse reactions in patients receiving Kuvan was similar to that reported with patients receiving placebo.
Because clinical trials were conducted under varying conditions, the observed adverse reaction rates may not predict the rates observed in patients in clinical practice. Table 1 enumerates treatment-emergent adverse reactions (regardless of relationship) that occurred in at least 4% of patients treated with Kuvan in the double-blind, placebo-controlled clinical trials described above. Reported frequency of adverse reactions was classified by MedDRA terms (Table 1).
| Treatment | ||
| Kuvan | Placebo | |
| Patients Treated | N=74 | N=59 |
| Preferred Term | N (%) | N (%) |
| Any Adverse Reaction | 47 (64) | 42 (71) |
| Headache | 11 (15) | 8 (14) |
| Upper respiratory tract infection | 9 (12) | 14 (24) |
| Rhinorrhea | 8 (11) | 0 |
| Pharyngolaryngeal pain | 7(10) | 1 (2) |
| Diarrhea | 6 (8) | 3 (5) |
| Vomiting | 6 (8) | 4 (7) |
| Cough | 5 (7) | 3 (5) |
| Pyrexia | 5 (7) | 4 (7) |
| Contusion | 4 (5) | 1 (2) |
| Abdominal pain | 4 (5) | 5 (8) |
| Rash | 4 (5) | 4 (7) |
| Nasal congestion | 3 (4) | 0 |
In open-label, uncontrolled clinical trials in which all patients received Kuvan in doses of 5 to 20 mg/kg/day, adverse reactions were similar in type and frequency to those reported in the double-blind, placebo-controlled clinical trials.
6.2 Safety Experience From Clinical Studies for Non-PKU Indications
Approximately 800 healthy volunteers and patients with disorders other than PKU, some of whom had underlying neurologic disorders or cardiovascular disease, have been administered a different formulation of the same active ingredient (sapropterin) in approximately 19 controlled and uncontrolled clinical trials. In these clinical trials, subjects were administered sapropterin at doses ranging from 1 to 20 mg/kg/day for lengths of exposure from 1 day to 2 years. Serious and severe adverse reactions (regardless of relationship) during sapropterin administration were convulsions, exacerbation of convulsions [seeWarnings and Precautions (5.8)], dizziness, gastrointestinal bleeding, post-procedural bleeding, headache, irritability, myocardial infarction, overstimulation, and respiratory failure. Common adverse reactions were headache, peripheral edema, arthralgia, polyuria, agitation, dizziness, and upper respiratory tract infection.
6.3 Post-Marketing Experience
The following adverse reactions have been identified during a 10-year post-approval safety surveillance program in Japan of another formulation of the same active ingredient (sapropterin). This safety surveillance program was conducted in 30 patients, 27 of whom had disorders other than PKU and had an underlying neurologic condition. The most common adverse reactions were convulsions and exacerbation of convulsions in 3 of the non-PKU patients [seeWarnings and Precautions (5.8)] and increased gamma-glutamyltransferase (GGT) in 2 of the non-PKU patients.
7 Drug Interactions
No drug interaction studies were performed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Women who are exposed to Kuvan during pregnancy are encouraged to enroll in the Kuvan patient registry [seePatient Counseling Information (17.5)].
Teratogenicity studies with sapropterin have been conducted in rats at oral doses up to 400 mg/kg/day (about 3 times the maximum recommended human dose of 20 mg/kg/day, based on body surface area) and in rabbits at oral doses of up to 600 mg/kg/day (about 10 times the maximum recommended human dose, based on body surface area). No clear evidence of teratogenic activity was found in either species; however, in the rabbit teratogenicity study, there was an increase (not statistically significant) in the incidence of holoprosencephaly at the 600 mg/kg/day dose compared to controls.
There are no adequate and well-controlled studies of Kuvan in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. A study of 468 pregnancies and 331 live births in PKU-effected women (Maternal Phenylketonuria Collaborative Study, Rouse 1997) has demonstrated that uncontrolled Phe levels above 600 µmol/L are associated with a very high incidence of neurological, cardiac, facial dysmorphism, and growth anomalies. Good dietary control of Phe levels during pregnancy is essential in reducing the incidence of Phe-induced teratogenic effects.
8.2 Labor and Delivery
The effects of Kuvan on labor and delivery in pregnant women are unknown.
8.3 Nursing Mothers
Sapropterin is excreted in the milk of intravenously, but not orally treated lactating rats. It is not known whether sapropterin is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from sapropterin and because of the potential for tumorigenicity shown for sapropterin in the rat carcinogenicity study, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Pediatric patients with PKU, ages 4 to 16 years, have been treated with Kuvan in clinical studies [seeClinical Studies (14.1)]. The safety and efficacy of Kuvan in pediatric patients less than 4 years of age have not been assessed in clinical studies. Frequent blood monitoring is recommended in the pediatric population to ensure adequate blood Phe level control [seePatient Counseling Information (17.2)].
8.5 Geriatric Use
Clinical studies of Kuvan in patients with PKU did not include patients aged 65 years and older. It is not known whether these patients respond differently than younger patients.
8.6 Patients With Renal Impairment
Patients with renal impairment have not been evaluated in clinical trials. Patients who have renal impairment should be carefully monitored when receiving Kuvan.
10 OVERDOSAGE
In the only reported overdosage with Kuvan, a patient participating in a 26-week study received a single dose of 4,500 mg (36 mg/kg) instead of 2,600 mg (20 mg/kg) in Week 16. The patient reported mild headache and mild dizziness immediately after taking the dose; both symptoms resolved within 1 hour with no treatment intervention. Results from liver function laboratory tests obtained immediately following the event were within normal limits. The patient suspended therapy for 24 hours and then restarted Kuvan with no reports of abnormal signs or symptoms.
11 DESCRIPTION
Sapropterin dihydrochloride, the active pharmaceutical ingredient in Kuvan Tablets, is a synthetic preparation of the dihydrochloride salt of naturally occurring tetrahydrobiopterin (BH4). Sapropterin dihydrochloride is an off-white to light yellow crystals or crystalline powder.
The chemical name of sapropterin dihydrochloride is (6R)-2-amino-6-[(1R,2S)-1,2-dihydroxypropyl]-5,6,7,8-tetrahydro-4(1H)-pteridinone dihydrochloride and the molecular formula is C9H15N5O3·2HCl with a molecular weight of 314.17.
Sapropterin dihydrochloride has the following structural formula:
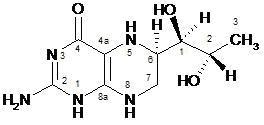
• 2 HCl
Each Kuvan Tablet contains 100 mg of sapropterin dihydrochloride (equivalent to 76.8 mg of sapropterin base). Tablets are round, off-white to light yellow, mottled, and debossed with “177”. Each tablet contains the following inactive ingredients: ascorbic acid (USP), crospovidone (NF), dibasic calcium phosphate (USP), D-mannitol (USP), riboflavin (USP), and sodium stearyl fumarate (NF).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Kuvan is a synthetic form of BH4, the cofactor for the enzyme phenylalanine hydroxylase (PAH). PAH hydroxylates Phe through an oxidative reaction to form tyrosine. In patients with PKU, PAH activity is absent or deficient. Treatment with BH4 can activate residual PAH enzyme, improve the normal oxidative metabolism of Phe, and decrease Phe levels in some patients.
12.2 Pharmacodynamics
In PKU patients who are responsive to BH4 treatment, blood Phe levels decrease within 24 hours after a single administration of sapropterin dihydrochloride, although maximal effect on Phe level may take up to a month, depending on the patient. A single daily dose of Kuvan is adequate to maintain stable blood Phe levels over a 24-hour period. Twelve patients with blood Phe levels ranging from 516 to 986 μmol/L (mean 747±153 μmol/L) were assessed with 24-hour blood Phe level monitoring following a daily morning dose of 10 mg/kg/day. The blood Phe level remained stable during a 24-hour observation period. No substantial increases in blood Phe levels were observed following food intake throughout the 24-hour period.
Doses above 20 mg/kg/day have not been evaluated in clinical studies.
12.3 Pharmacokinetics
Studies in healthy volunteers have shown comparable absorption of sapropterin dihydrochloride when tablets are dissolved in water or orange juice and taken under fasted conditions. Administration of dissolved tablets after a high-fat/high-calorie meal resulted in mean increases in Cmax of 84% and AUC of 87% (dissolved in water). However, there was extensive variability in individual subject values for Cmax and AUC across the different modes of administration and meal conditions. In the clinical trials of Kuvan, drug was administered in the morning as a dissolved tablet without regard to meals. The mean elimination half-life in PKU patients was approximately 6.7 hours (range 3.9 to 17 hr), comparable with values seen in healthy subjects (range 3.0 to 5.3 hr).
A population pharmacokinetic analysis of sapropterin that included patients between 9 and 49 years of age showed no effect of age on sapropterin dihydrochloride pharmacokinetics. Pharmacokinetics in patients <9 years and >49 years of age have not been studied.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year carcinogenicity study was conducted in F-344 rats, and a 78-week carcinogenicity study was conducted in CD-1 mice. In the 104-week oral carcinogenicity study in rats, sapropterin doses of 25, 80, and 250 mg/kg/day (0.2, 0.7, and 2 times the maximum recommended human dose of 20 mg/kg/day, respectively, based on body surface area) were used. In the 78-week oral carcinogenicity study in mice, sapropterin doses of 25, 80, and 250 mg/kg/day (0.1, 0.3, and 2 times the recommended human dose, respectively, based on body surface area) were used. In the 2-year rat carcinogenicity study, there was a statistically significant increase in the incidence of benign adrenal pheochromocytoma in male rats treated with the 250 mg/kg/day (about 2 times the maximum recommended human dose, based on body surface area) dose, as compared to vehicle-treated rats. The mouse carcinogenicity study showed no evidence of a carcinogenic effect, but the study was not ideal due to its duration of 78 instead of 104 weeks.
Sapropterin was genotoxic in the in vitro Ames test at concentrations of 625 µg (TA98) and 5000 µg (TA100) per plate, without metabolic activation. However, no genotoxicity was observed in the in vitro Ames test with metabolic activation. Sapropterin was genotoxic in the in vitro chromosomal aberration assay in Chinese hamster lung cells at concentrations of 0.25 and 0.5 mM. Sapropterin was not mutagenic in the in vivo micronucleus assay in mice at doses up to 2000 mg/kg/day (about 8 times the maximum recommended human dose of 20 mg/kg/day, based on body surface area). Sapropterin, at oral doses up to 400 mg/kg/day (about 3 times the maximum recommended human dose, based on body surface area) was found to have no effect on fertility and reproductive function of male and female rats.
14 CLINICAL STUDIES
14.1 Clinical Studies in PKU
The efficacy and safety of Kuvan were evaluated in 4 clinical studies in patients with PKU.
Study 1 was a multicenter, open-label, uncontrolled clinical trial of 489 patients with PKU, ages 8 to 48 years (mean 22 years), who had baseline blood Phe levels ≥450 μmol/L and who were not on Phe-restricted diets. All patients received treatment with Kuvan 10 mg/kg/day for 8 days. For the purposes of this study, response to Kuvan treatment was defined as a ≥30% decrease in blood Phe from baseline. At Day 8, 96 patients (20%) were identified as responders.
Study 2 was a multicenter, double-blind, placebo-controlled study of 88 patients with PKU who responded to Kuvan in Study 1. After a washout period from Study 1, patients were randomized equally to either Kuvan 10 mg/kg/day (N=41) or placebo (N=47) for 6 weeks. Efficacy was assessed by the mean change in blood Phe level from baseline to Week 6 in the Kuvan-treated group as compared to the mean change in the placebo group.
The results showed that at baseline, the mean (±SD) blood Phe level was 843 (±300) μmol/L in the Kuvan-treated group and 888 (±323) μmol/L in the placebo group. At Week 6, the Kuvan-treated group had a mean (±SD) blood Phe level of 607 (±377) μmol/L, and the placebo group had a mean blood Phe level of 891 (±348) μmol/L. At Week 6, the Kuvan- and placebo-treated groups had mean changes in blood Phe level of ―239 and 6 μmol/L, respectively (mean percent changes of ―29% (±32) and 3% (±33), respectively). The difference between the groups was statistically significant (p<0.001) (Table 2).
| Sapropterin (N=41) | Placebo (N=47) | |
| Baseline Blood Phe Level1 (μmol/L) | ||
| Mean (±SD) | 843 (±300) | 888 (±323) |
| Percentiles (25th , 75th) | 620, 990 | 618, 1141 |
| Week 6 Blood Phe Level (μmol/L) | ||
| Mean (±SD) | 607 (±377) | 891 (±348) |
| Percentiles (25th , 75th) | 307, 812 | 619, 1143 |
| Mean Change in Blood Phe From Baseline to Week 6 (μmol/L) | ||
| Adjusted Mean (±SE)2 | -239 (±38) | 6 (±36) |
| Percentiles (25th , 75th) | -397, -92 | -96, 93 |
| Mean Percent Change in Blood Phe From Baseline to Week 6 | ||
| Mean (±SD) | - 29 (±32) | 3 (±33) |
| Percentiles (25th , 75th) | -61, -11 | -13, 12 |
1The mean baseline (BL) levels shown in this table represent the mean of 3 pretreatment levels (Wk -2, Wk -1, and Wk 0). Treatment with Kuvan or placebo started at Wk 0.
2p-value < 0.001, adjusted mean and standard error from an ANCOVA model with change in blood Phe level from baseline to Week 6 as the response variable, and both treatment group and baseline blood Phe level as covariates.
Change in blood Phe was noted in the Kuvan-treated group at Week 1 and was sustained through Week 6 (Figure 1).
Figure 1: Mean Blood Phenylalanine (Phe) Level Over Time1
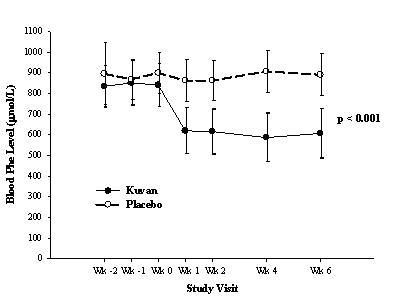
1Error bars indicate 95% confidence interval.
Study 3 was a multicenter, open-label, extension study in which 80 patients who responded to Kuvan treatment in Study 1 and completed Study 2 underwent 6 weeks of forced dose-titration with 3 different doses of Kuvan. Treatments consisted of 3 consecutive 2 week courses of Kuvan at doses of 5, then 20, and then 10 mg/kg/day. Blood Phe level was monitored after 2weeks of treatment at each dose level. At baseline, mean (±SD) blood Phe was 844 (±398) μmol/L. At the end of treatment with 5, 10, and 20 mg/kg/day, mean (±SD) blood Phe levels were 744 (±384) μmol/L, 640 (±382) μmol/L, and 581 (±399) μmol/L, respectively (Table 3).
| Kuvan Dose Level (mg/kg/day) | No. of Patients | Mean (±SD) Blood Phe Level (μmol/L) | Mean Changes (±SD) in Blood Phe Level From Week 0 (μmol/L) |
| Baseline(No Treatment) | 80 | 844 (±398) | − |
| 5 | 80 | 744 (±384) | -100 (±295) |
| 10 | 80 | 640 (±382) | -204 (±303) |
| 20 | 80 | 581 (±399) | -263 (±318) |
Study 4 was a multicenter study of 90 children with PKU, ages 4 to 12 years, who were on Phe-restricted diets and who had blood Phe levels ≤480 μmol/L at screening. All patients were treated with open-label Kuvan 20 mg/kg/day for 8 days. Response to Kuvan was defined as a ≥30% decrease in blood Phe from baseline at Day 8. At Day 8, 50 patients (56%) had a ≥30% decrease in blood Phe.
16. How Supplied/Storage and Handling
Kuvan TM (sapropterin dihydrochloride) Tablets are supplied in high-density polyethylene bottles, sealed with aluminized film, and closed with child-resistant caps. Each bottle contains 30 or 120 tablets, a silica gel desiccant cartridge, and a pharmaceutical-grade polyester coil. Each Kuvan Tablet contains 100 mg of sapropterin dihydrochloride (equivalent to 76.8 mg of sapropterin base).
Bottle of 30 tablets……………………………………………………...NDC 68135-300-01
Bottle of 120 tablets…………………………………………………….NDC 68135-300-02
Storage
Store at 20°C to 25°C (68-77°F); excursions allowed between 15°C to 30°C (59-86°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from moisture.
Rx Only
Manufactured for: BioMarin Pharmaceutical Inc.
Novato, CA 94949
Manufactured by: Lyne Laboratories, Inc.
Brockton, MA 02301
17 Patient Counseling Information
See FDA-Approved Patient Information Labeling (17.6).
Patients should be advised of the following information before beginning treatment with Kuvan:
17.1 Important Information to Consider Prior to Prescribing Kuvan
Patients with residual PAH enzyme activity may benefit from taking Kuvan; however, not all patients with PKU respond to treatment with Kuvan. In clinical trials, approximately 20% to 56% of PKU patients responded to treatment with Kuvan, and reductions in blood Phe levels were observed in patients across the continuum of PKU phenotypes (mild, moderate, and severe PKU).
Since patients have varying degrees of residual PAH enzyme activity and BH4 responsiveness, it is not possible to accurately predict the extent of response before administering Kuvan to the patient, and response to treatment cannot be pre-determined by laboratory testing (e.g., genetic testing). Response to Kuvan can only be determined by a therapeutic trial.
To determine if a patient may respond to treatment with Kuvan, the patient must be treated with Kuvan and evaluated for changes in blood Phe. Blood Phe levels and dietary Phe intake should be measured frequently [seeWarnings and Precautions (5.2 and 5.3)].
17.2 Blood Phe Monitoring and Management
Treatment with Kuvan should be directed by physicians knowledgeable in the management of PKU, and the initiation of Kuvan therapy does not eliminate the need for appropriate monitoring by trained professionals. Patients being treated with Kuvan should have frequent blood Phe measurements and nutritional counseling with their physician and other members of the health care team to ensure maintenance of blood Phe levels in the desirable range.
Since changes in dietary Phe intake can obscure the effect of Kuvan on blood Phe levels, and since not all patients will respond to treatment with Kuvan, all patients with PKU should be treated with a Phe-restricted diet in addition to treatment with Kuvan [seeWarnings and Precautions (5.3)].
To determine if a patient responds to Kuvan therapy, patients must not modify their existing dietary Phe intake during the evaluation period in order to get an accurate assessment of the effect of Kuvan on blood Phe levels. Baseline blood Phe measurements should be taken just prior to initiation of a Kuvan response test. Patients should be started at a dose of 10 mg/kg/day. Blood Phe levels should be checked after 1 week of Kuvan treatment and periodically for up to a month to determine response. A response to treatment with Kuvan may be determined by a decrease in blood Phe level compared to baseline level. If blood Phe level does not decrease at 10 mg/kg/day, the dose may be increased to 20 mg/kg/day. Patients whose blood Phe does not decrease from baseline after 1 month of treatment at 20 mg/kg/day are non-responders, and treatment with Kuvan should be discontinued in these patients [seeDosage and Administration (2.1)].
For patients who respond to Kuvan treatment, the dosage may be adjusted within the range of 5 to 20 mg/kg/day according to response to therapy. Doses above 20 mg/kg/day have not been evaluated in clinical trials.
After the dose of Kuvan has been established, continued active management of dietary Phe intake using medical foods and natural sources of proteins is required to ensure blood Phe control and adequate nutritional balance.
17.3 What Are the Benefits of Taking Kuvan?
Prolonged high blood Phe levels are neurotoxic and lead to impairment of intelligence and other brain functions (such as attentiveness). Reduction of blood Phe levels through dietary control is an important determinant of long-term neurologic outcome in PKU patients, and reduction of blood Phe levels in patients with PKU has been shown to decrease the long-term risk of neurologic injury. It is difficult for many patients to maintain reduced blood Phe, and many patients with PKU experience some degree of neurological impairment despite efforts to maintain dietary Phe control.
In clinical trials with Kuvan in patients with PKU, reductions in blood Phe levels were observed in some patients. Although long-term assessment of neurologic function in patients with PKU receiving Kuvan for the treatment of elevated blood Phe has not been assessed, Kuvan may help maintain reduced blood Phe levels as an adjunct to a Phe-controlled diet.
17.4 What Are the Risks of Taking Kuvan?
Response to Kuvan treatment in PKU patients is variable. Not all patients responded to treatment with Kuvan in clinical trials, and the initiation of Kuvan treatment does not eliminate the need to monitor for adequate blood Phe control. Prolonged elevations in blood Phe levels can result in neurologic impairment. Conversely, some patients in clinical trials who were following Phe-restricted diets and received treatment with Kuvan experienced substantial reductions of blood Phe. Levels of blood Phe that are too low may be associated with catabolism and protein breakdown. Therefore, when Kuvan is used in combination with a Phe-restricted diet, patients should be monitored closely to ensure that blood Phe levels are not too low, and, if necessary, the dose of Kuvan should be adjusted.
The most serious adverse reactions during Kuvan administration (regardless of relationship to treatment) were gastritis, spinal cord injury, streptococcal infection, testicular carcinoma, and urinary tract infection. Mild to moderate neutropenia was noted during Kuvan administration in 24 of 579 patients (4%). The most common (≥4% of patients treated with Kuvan) across all studies (n=579) were headache, diarrhea, abdominal pain, upper respiratory tract infection, pharyngolaryngeal pain, vomiting, and nausea [seeAdverse Reactions (6.1)].
Long-term studies of neurocognitive outcomes have not been conducted with Kuvan.
17.5 BioMarin PKU Disease Registries
BioMarin will establish a general disease registry for PKU patients and a pregnancy registry for women who are pregnant while receiving Kuvan treatment.
17.6 FDA-Approved Patient Information Labeling
PHARMACIST— DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
PATIENT INFORMATION
For people with PKU
Kuvan (COO-van)
(sapropterin dihydrochloride) Tablets
Read this leaflet before you start taking Kuvan and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
What is Kuvan?
Kuvan is a medicine for people with Phenylketonuria (PKU). An enzyme in your body PAH (phenylalanine hydroxylase) helps break down phenylalanine (Phe), an amino acid found in food. In patients with PKU this enzyme does not work right. PKU leads to high blood Phe levels. High blood Phe levels are toxic to the brain and can lead to lower intelligence and decrease in the ability to focus, remember, and organize information.
How does Kuvan work?
Kuvan acts in your body with the enzyme PAH to reduce your blood Phe levels. Your doctor and health care team will continue to monitor your blood Phe levels and dietary Phe intake.
Kuvan are tablets that you should dissolve in water or apple juice before taking.
Who may benefit from taking Kuvan?
It is not possible to know whether or not Kuvan will work for you until you start taking Kuvan. Your doctor will monitor your blood Phe levels when you start taking Kuvan to see if the drug is working.
What are the risks of taking Kuvan?
When you are taking Kuvan, any change you make to your diet may affect your blood Phe level. Follow your doctor’s instructions carefully and do not make any changes to your dietary Phe intake before discussing with your doctor. Your doctor will continue to monitor your blood Phe levels during your treatment with Kuvan.
If you have a fever, or if you are sick, your blood Phe level may go up. Tell your doctor as soon as possible so they can see if they have to adjust your treatment to help keep your blood Phe levels in the desired range.
What should I tell my doctor before taking Kuvan?
Before you start taking Kuvan, let your doctor know about all of your medical conditions, including if you:
-
Have a fever
-
Are pregnant or planning to become pregnant
-
Are breast feeding
-
Have liver problems
-
Are allergic to Kuvan or any other medications
-
Have poor nutrition or are anorexic
-
Are taking levodopa
-
Are taking drugs that inhibit folate metabolism (e.g., methotrexate) because these drugs could affect how Kuvan works in your body
-
Are taking medicines for erectile dysfunction like Viagra (sildenafil), Levitra (vardenafil), or Cialis (tadalafil)
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines and herbal and dietary supplements. Kuvan and many other medicines may interact with each other. Your doctor needs to know what medicines you take so he or she can decide if Kuvan is right for you.
Know the medicines you take. Keep a list of your medicines with you to show your doctor. Do not take other medicines while taking Kuvan without first talking to your doctor.
How should I take Kuvan?
Kuvan Tablets are taken at one time each day. Take Kuvan exactly as your doctor has told you.
-
Take Kuvan once a day with food and preferably at the same time each day.
-
Kuvan Tablets should be dissolved in 4 to 8 ounces (1/2 to 1 cup) of water or apple juice.
-
To dissolve the tablets, mix them in water or apple juice, and drink within 15 minutes.
-
It may take a few minutes for the tablets to dissolve. To make the tablets dissolve faster, you can stir or crush them.
-
The tablets may not dissolve completely. You may see small pieces floating on top of the water or apple juice. This is normal and safe for you to swallow.
-
If after drinking your medicine you still see small pieces of the tablet, you should add more water or apple juice to make sure that you take all of your medicine.
-
-
If you forget to take your dose of Kuvan, take it as soon as you remember that day. If you miss a day, do not double your dose the next day, just skip the missed dose.
-
The recommended starting dose of Kuvan is 10 mg/kg taken once a day. Your doctor will tell you the dose you should take and when to take it.
-
Your doctor can change your dose depending on how you respond to treatment.
What are the possible side effects of Kuvan?
The most common side effects reported when using Kuvan are:
-
Headache
-
Diarrhea
-
Abdominal pain
-
Upper respiratory tract infection (like a cold)
-
Throat pain
-
Vomiting
-
Nausea
These are not all the side effects seen with Kuvan. If you are concerned about these or any other side effects you experience while taking Kuvan, ask your doctor or pharmacist for more information.
Be sure to tell your doctor if you have any side effects when you are taking Kuvan.
How should I store Kuvan?
-
Store in a cool, dry place between 68°F and 77°F (20-25°C).
-
Do not leave Kuvan in hot or humid places, such as your car or bathroom cabinet.
-
Keep Kuvan in its original bottle with the cap closed tightly.
-
Protect from moisture. Do not remove the dessicant (the small packet included with your tablets). The dessicant absorbs moisture.
-
The color of the tablets may change over time to light yellow. This is normal and you can take these tablets.
-
Do not keep Kuvan that is out of date, or that you no longer need. Be sure that if you throw any medicine away, it is out of the reach of children.
-
Keep Kuvan and all medicines out of the reach of children.
General information about Kuvan
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Kuvan for any other condition. Do not give Kuvan to anyone else, even if they have the same condition that you have. It may harm them.
This leaflet summarizes the most important information about Kuvan. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Kuvan that is written for health professionals. For more information you can call BioMarin Patient and Physician Support (BPPS) for free at 1-866-906-6100.
What are the ingredients in Kuvan?
Active Ingredient: sapropterin dihydrochloride.
Inactive Ingredients: ascorbic acid, crospovidone, dibasic calcium phosphate, D-mannitol, riboflavin, and sodium stearyl fumarate.
Kuvan Tablets are mottled, off-white to light yellow, and debossed with “177”.

Kuvan is a trademark of BioMarin Pharmaceutical Inc.

BioMarin Pharmaceutical Inc.
Novato, CA 94949
© BioMarin Pharmaceutical Inc. All rights reserved.
PACKAGE LABEL

NDC 68135-300-01
KUVAN™
(sapropterin dihydrochloride) Tablets
100 mg*
* Equivalent to 76.8 mg of sapropterin
Rx only
30 Tablets
BiOMARIN®
Manufactured for
BioMarin Pharmaceutical Inc.
Novato, CA 94949
by Lyne Laboratories, Inc.
Brocton, MA 02301
Store at 20°C to 25°C (68°F-77°F); excursions allowed between 15°C to 30°C (59°F-86°F)
[See USP Controlled Room Temperature]
Keep container tightly closed
Protect from moisture
Usual Dosage:
See Physician Package Insert
T1419001C (12/07)
Lot:
Exp:

NDC 68135-300-01
KUVAN™
(sapropterin dihydrochloride) Tablets
100 mg*
* Equivalent to 76.8 mg of sapropterin
Rx only
30 Tablets
BiOMARIN®
1777 T1419001 (12/07)
Store at 20°C to 25°C (68°F-77°F); excursions allowed between 15°C to 30°C (59°F-86°F)
[See USP Controlled Room Temperature]
Keep container tightly closed
Protect from moisture
Usual Dosage: See Physician Package Insert
Manufactured for
BioMarin Pharmaceutical Inc. Novato, CA 94949
by Lyne Laboratories, Inc. Brocton, MA 02301
| KUVAN
sapropterin dihydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022181 | 12/14/2007 | |
| Labeler - BioMarin Pharmaceutical Inc. (007004745) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| BioMarin Pharmaceutical Inc. | 010004135 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LYNE LABORATORIES, INC. | 053510459 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| EXCELLA GMBH | 329809800 | MANUFACTURE | |