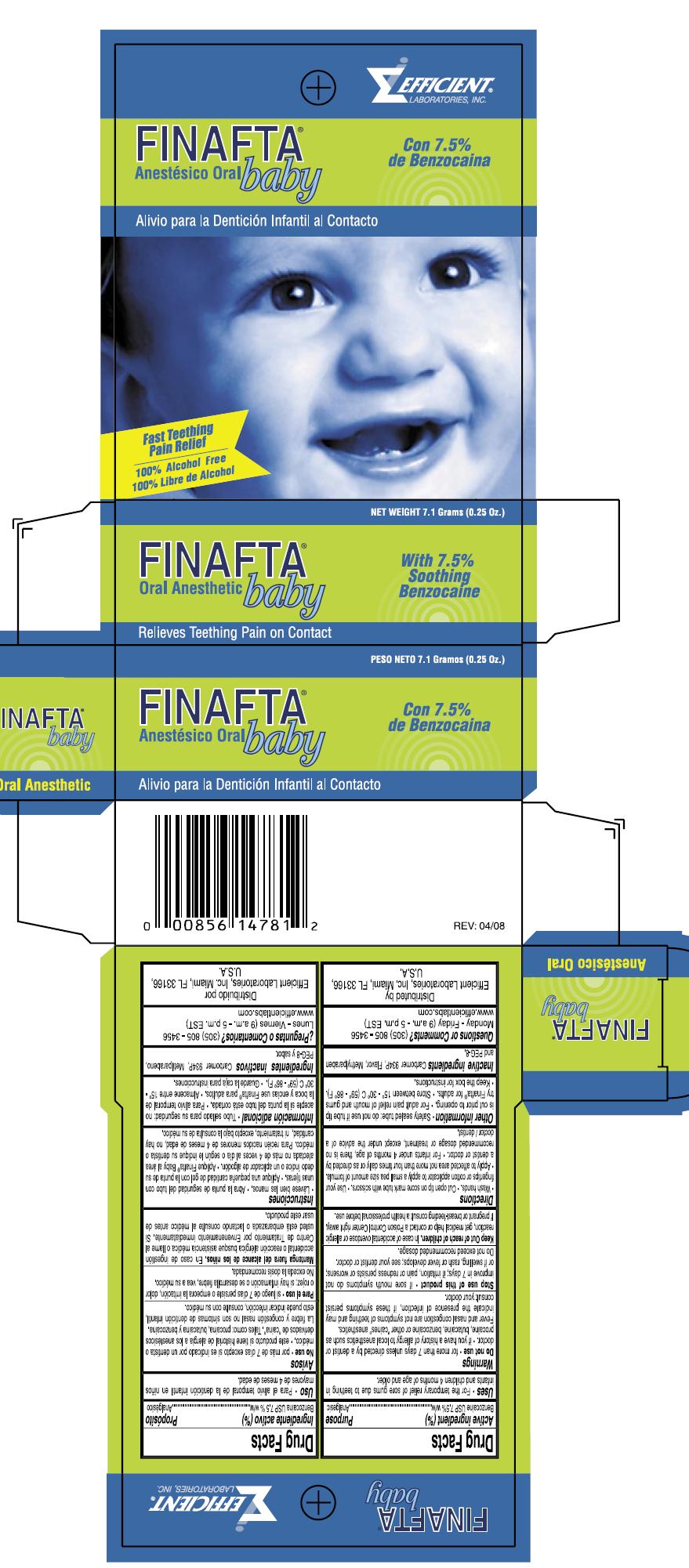
FINAFTA BABY- benzocaine liquid
Efficient Laboratories Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
For the temporary relief of sore gums due to teething in infants and children 4 months of age and older.
Warnings
Allergy Alert:Do not use this product if your baby has a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or ther "caine" anesthetics.
Do not use for more than 7 days unless directed by a dentist or doctor
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection, if these symptoms do not go away, advise your dentist or doctor.
Keep out of reach of children. In case of accidental overdose or allergic reaction, get medical help or contact a Poison Control Center right away.
Wash hands. Cut open tip on score mark with scissors Use your fingertips or cotton applicator to apply a small pea size amount of formula. Apply to affected area not more than four times daily or as directed by a dentist or doctor For infants under 4 months of age, these is no recommended dosage or treatment, except under the advice of a doctor/dentist
Safety sealed tube: do not use if tube tip is cut prior to opening Store between 15-30 degrees C (59-86 degrees F). Keep the box for instructions
| FINAFTA
BABY
benzocaine liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Efficient Laboratories Inc. (969044932) |