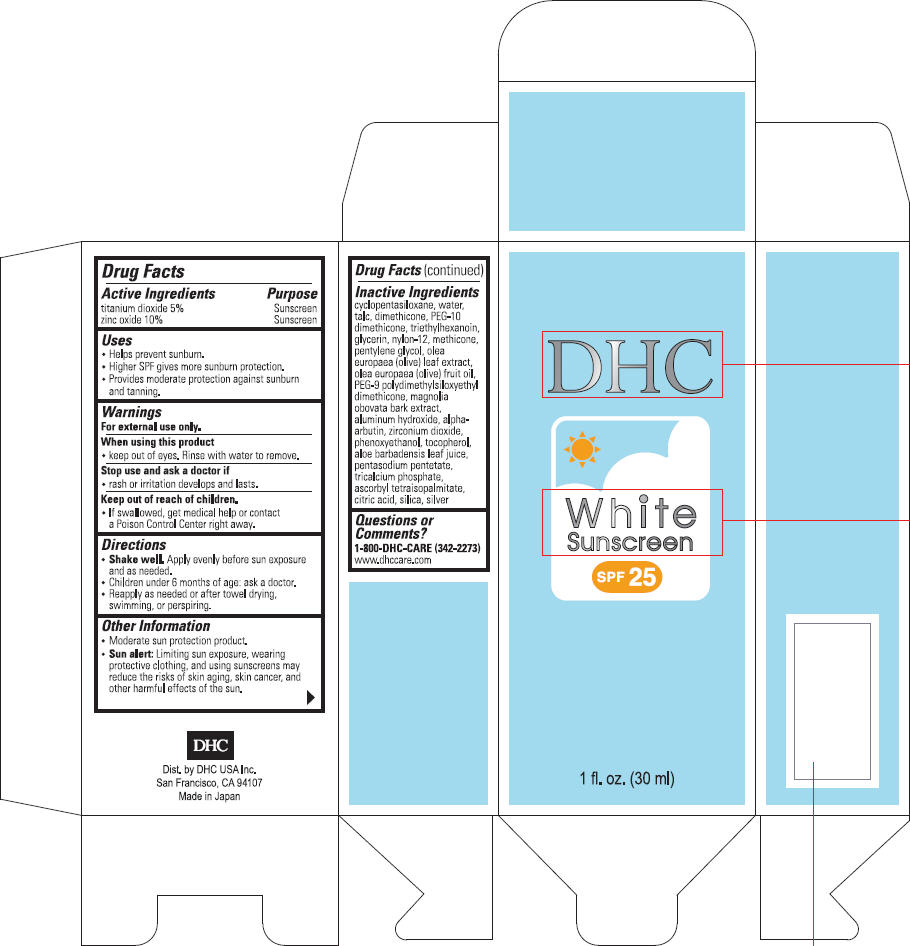
DHC WHITE SUNSCREEN
-
titanium dioxide and
zinc oxide cream
DHC USA Incorporated
----------
DHCWhite
Sunscreen
Drug Facts
| Active Ingredients | Purpose |
|---|---|
| titanium dioxide 5% | Sunscreen |
| zinc oxide 10% | Sunscreen |
Uses
- Helps prevent sunburn.
- Higher SPF gives more sunburn protection.
- Provides moderate protection against sunburn and tanning.
Warnings
For external use only.
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Shake well. Apply evenly before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- Reapply as needed or after towel drying, swimming, or perspiring.
Other Information
- Moderate sun protection product.
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
Inactive Ingredients
cyclopentasiloxane, water, talc, dimethicone, PEG-10 dimethicone, triethylhexanoin, glycerin, nylon-12, methicone, pentylene glycol, olea europaea (olive) leaf extract, olea europaea (olive) fruit oil, PEG-9 polydimethylsiloxyethyl dimethicone, magnolia obovata bark extract, aluminum hydroxide, alpha-arbutin, zirconium dioxide, phenoxyethanol, tocopherol, aloe barbadensis leaf juice, pentasodium pentetate, tricalcium phosphate, ascorbyl tetraisopalmitate, citric acid, silica, silver
Questions or Comments?
1-800-DHC-CARE (342-2273)
www.dhccare.com
PRINCIPAL DISPLAY PANEL - 30 ml Carton
DHC
White
Sunscreen
SPF 25
1 fl. oz. (30 ml)
| DHC WHITE SUNSCREEN
titanium dioxide and zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 05/01/2005 | |
| Labeler - DHC USA Incorporated (004087554) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Picaso Cosmetic Laboratory Limited | 718552578 | MANUFACTURE, LABEL, PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DHC USA Incorporated | 010058429 | REPACK | |