AK-CON
-
naphazoline hydrochloride solution
Preferred Pharmaceuticals, Inc
----------
ALBALON®(naphazoline hydrochloride ophthalmic solution, USP) 0.1%
Sterile
DESCRIPTION
Naphazoline hydrochloride, an ocular vasoconstrictor, is an imidazoline derivative sympathomimetic amine. It occurs as a white, odorless crystalline powder having a bitter taste and is freely soluble in water and in alcohol. The active ingredient is represented by the structural formula:
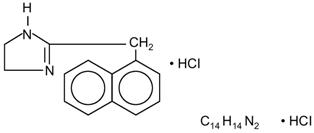
Chemical Name:
2-(1-Naphthylmethyl)-2-imidazoline monohydrochloride
Contains:
Active: naphazoline HCl 1 mg (0.1%). Preservative: benzalkonium chloride 0.1mg (0.01%).
Inactives: Boric acid; edetate disodium; purified water; sodium chloride; sodium carbonate; and hydrochloric acid may be added to adjust the pH (5.5 to 7.0).
CLINICAL PHARMACOLOGY
Naphazoline constricts the vascular system of the conjunctiva. It is presumed that this effect is due to direct stimulation of the drug upon the alpha-adrenergic receptors in the arterioles of the conjunctiva, resulting in decreased conjunctival congestion. Naphazoline belongs to the imidazoline class of sympathomimetics.
INDICATIONS AND USAGE
Naphazoline Hydrochloride Ophthalmic Solution is indicated for use as a topical ocular vasoconstrictor.
CONTRAINDICATIONS
Contraindicated in the presence of an anatomically narrow angle or in narrow-angle glaucoma or in persons who have shown hypersensitivity to any component of this preparation.
WARNINGS
Patients under therapy with MAO inhibitors may experience a severe hypertensive crisis if given a sympathomimetic drug. Use in children, especially infants, may result in CNS depression leading to coma and marked reduction in body temperature.
PRECAUTIONS
General:
For topical ophthalmic use only. Use with caution in the presence of hypertension, cardiovascular abnormalities, hyperglycemia (diabetes), hyperthyroidism, infection or injury.
Patient Information:
Patients should be advised to discontinue the drug and consult a physician if relief is not obtained within 48 hours of therapy, if irritation, blurring or redness persists or increases, or if symptoms of systemic absorption occur, i.e., dizziness, headache, nausea, decrease in body temperature, or drowsiness.
To prevent contaminating the dropper tip and solution, do not touch the eyelids or the surrounding area with the dropper tip of the bottle. If solution changes color or becomes cloudy, do not use.
Drug Interactions:
Concurrent use of maprotiline or tricyclic antidepressants and naphazoline may potentiate the pressor effect of naphazoline. Patients under therapy with MAO inhibitors may experience a severe hypertensive crisis if given a sympathomimetic drug. (See WARNINGS.)
Pregnancy:
Pregnancy Category C: Animal reproduction studies have not been
conducted with naphazoline. It is also not known whether naphazoline can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Naphazoline should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether naphazoline is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when naphazoline is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established. See “WARNINGS.” and “CONTRAINDICATIONS.”
ADVERSE REACTIONS
Ocular: Mydriasis, increased redness, irritation, discomfort, blurring, punctate keratitis, lacrimation, increased intraocular pressure.
Systemic: Dizziness, headache, nausea, sweating , nervousness, drowsiness, weakness, hypertension, cardiac irregularities, and hyperglycemia.
DOSAGE AND ADMINISTRATION
Instill one or two drops in the conjunctival sac(s) every three to four hours as needed.
HOW SUPPLIED
Naphazoline Hydrochloride Ophthalmic Solution, USP) is supplied as a sterile 0.1% solution in
15 mL plastic dropper bottles. NDC 17478-216-12
Storage: Store at 20° to 25°C (68° to 77°F). Keep container tightly closed.
Rx Only
Manufactured by:
Akorn Inc.
Lake Forest, IL 60045
Repackaged by:
Preferred Pharmaceuticals, IncAnaheim, CA
Principal Display Panel
| AK-CON
naphazoline hydrochloride solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA083590 | 08/22/1974 | |
| Labeler - Preferred Pharmaceuticals, Inc (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Preferred Pharmaceuticals, Inc | 791119022 | RELABEL, REPACK | |