DG HEALTH PAIN RELIEF PM EXTRA STRENGTH
-
acetaminophen and
diphenhydramine hydrochloride tablet, film coated
Dolgencorp, LLC
----------
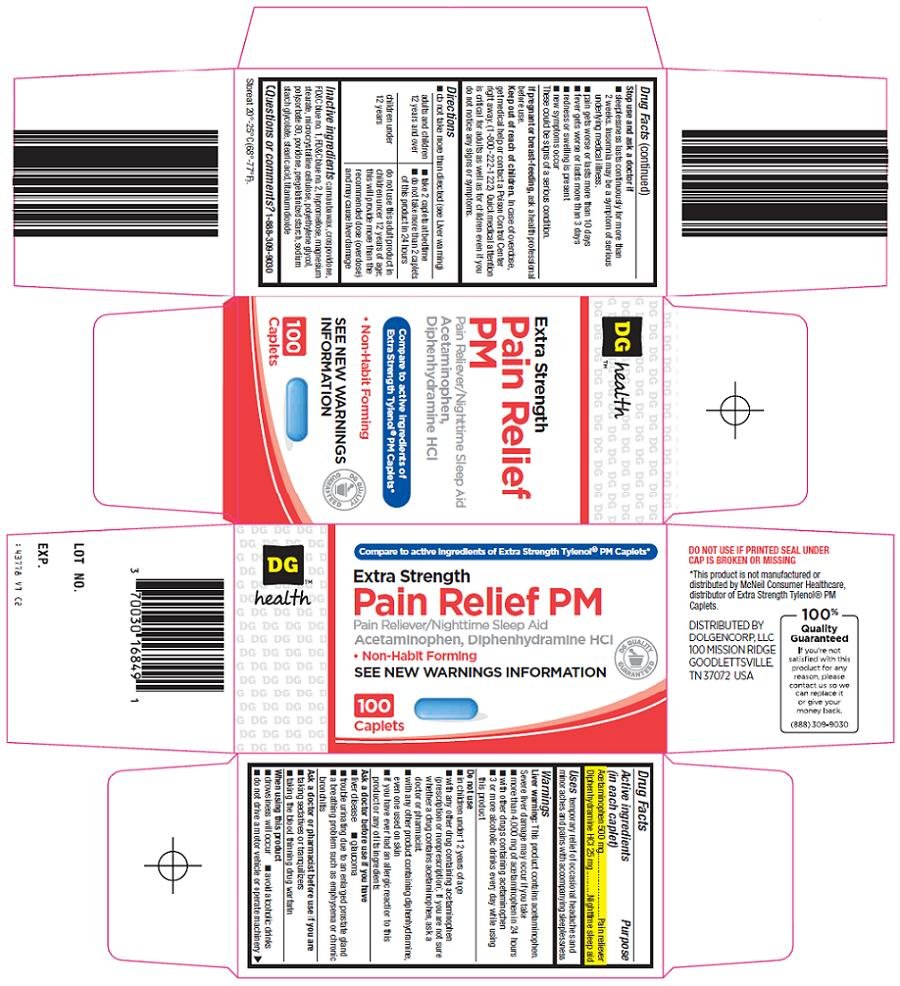
Dolgencorp, LLC Pain Relief PM Drug FactsActive ingredient (in each caplet)
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Purpose
Pain reliever
Nighttime sleep aid
Uses
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- in children under 12 years of age
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness lasts continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see Liver warning)
| adults and children 12 years and over |
|
| children under 12 years | do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and may cause liver damage |
Inactive ingredients
carnauba wax, crospovidone, FD&C blue no. 1, FD&C blue no. 2, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, stearic acid, titanium dioxide
Questions or comments?
1-888-309-9030
Principal Display Panel
Compare to active ingredients of Extra Strength Tylenol® PM Caplets
Extra Strength
Pain Relief PM
Pain Reliever/Nighttime Sleep Aid
Acetaminophen, Diphenhydramine HCl
Non-habit forming
See New Warnings Information
Pain Relief PM Carton
| DG HEALTH PAIN RELIEF PM
EXTRA STRENGTH
acetaminophen, diphenhydramine hcl tablet, film coated |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part338 | 11/21/2009 | |
| Labeler - Dolgencorp, LLC (068331990) |