COLCHICINE
-
colchicine tablet
Physicians Total Care, Inc.
----------
|
|||||||||||||||||||||||
| FULL PRESCRIBING INFORMATION: CONTENTS* | |
|---|---|
|
|
|
|
|
FULL PRESCRIBING INFORMATION
INDICATIONS & USAGE
Colchicine is indicated for prophylaxis of gout flares in adults
DOSAGE & ADMINISTRATION
The dosage for adults and adolescents older than 16 years of age is 0.6 mg (one tablet) once or twice daily. Maximum dose 1.2 mg/day.
Dosage reduction should be carefully considered in patients with impaired hepatic or renal function and in patients who are receiving concomitant treatment with P-gp and CYP3A4 inhibitors.
Colchicine tablets are administered orally, without regard to meals.
Colchicine is not an analgesic medication and should not be used to treat pain from other causes.
The safety and efficacy of colchicine in children to reduce the incidence of acute gout flares has not been evaluated.
DOSAGE FORMS & STRENGTHS
0.6 mg tablets ― white, round, unscored, shallow concave compressed tablets with WEST-WARD 201 debossed on tablet.
CONTRAINDICATIONS
Colchicine should not be prescribed to patients with a hypersensitivity to colchicine. Colchicine should not be prescribed to patients with impaired renal or hepatic function who are taking P-gp or strong CYP3A4 inhibitors..
WARNINGS AND PRECAUTIONS
General
In rare instances severe allergic reactions and anaphylaxis have been reported with the use of colchicine. Most of these rare allergic reactions have been reported to occur within several hours after readministration following prior usage of the drug. The appearance of hypersensitivity reactions requires cessation of therapy with colchicine.
Both accidental and intentional overdoses with colchicine in children and adults have been reported [See OVERDOSE (10)]. Keep Colchicine Tablets out of the reach of children.
Renal ImpairmentColchicine is significantly excreted in urine in healthy
subjects. Clearance of Colchicine is decreased in patients with impaired renal
function. Consideration should be given to the reduction of the dosage in
patients with renal impairment. Patients with any degree of renal dysfunction,
who are treated with Colchicine, should be monitored closely for adverse effects
of Colchicine.
The clearance of Colchicine may be significantly reduced and plasma half-life prolonged in patients with chronic hepatic impairment, compared to healthy subjects. Consideration should be given to the reduction of the dosage in patients with hepatic impairment. All patients with hepatic impairment should be monitored closely for adverse effects of Colchicine.
ADVERSE REACTIONS
The most frequent adverse effects of oral colchicine in therapeutic doses are those involving the gastrointestinal tract with diarrhea, nausea, vomiting, and abdominal pain often being the first signs of toxicity and the first indication that the colchicine dose may need to be reduced or therapy stopped. Larger doses may cause profuse diarrhea, gastrointestinal hemorrhage, skin rashes, renal and hepatic damage. Bone marrow suppression with agranulocytosis, thrombocytopenia, and aplastic anemia have rarely occurred with prolonged treatment, as have peripheral neuropathy, myopathy, rashes, and alopecia.
Side effects due to Colchicine appear to be a function of dosage. The possibility of increased colchicine toxicity in the presence of renal or hepatic dysfunction should be considered.
DRUG INTERACTIONS
Since colchicine is metabolized by cytochrome P (CYP) 3A4 demethylation in the liver, it may interact with substrates of this enzyme system, including estrogen, steroids, dapsone, diltiazem, erythromycin, lidocaine, lovastatin (and most other statins), midazolam, quinidine, terfenadine, testosterone, nifedipine, and verapamil. It may also be affected by inhibitors of this enzyme system, such as diltiazem, gestodene, grapefruit juice, ketoconazole, toleadomycin, and erythromycin. (Ben-Chetrit and Levy, 2003) (Hsu, Chen, Chang, and Chiu, 2002) (Holtzman, Wiggins, and Spinler, 2006)P-glycoprotein modulators or substrates that may interact with colchicine include morphine, doxorubicin, vinblastine, vincristine, paclitaxel, digoxin, quinidine, amprenavir, indinavir, nelfinavir, ritonavir, saquinavir, loperamide, josamycin, erythromycin, clarithromycin, cyclosporine, aldosterone, dexamethasone, prednisolone, progesterone, verapamil, talinolol, fexofenadine, cimetidine, amitriptyline, nortriptyline, phenytoin, and simvastatin. (Niel and Scherrmann, 2006)
Administration of colchicine with macrolide antibiotics such as clarithromycin impairs colchicine elimination, resulting in excess drug accumulation and toxicity, (Akdag, Erosy, Kahvecioglu, Gullulu, and Dilek, 2006) (Rollot, Pajot, Chauvelot-Moachon, Nazal, Kelaidi, and Blanche, 2004)Acute myopathy has been reported when colchicine is used with pravastatin. (Alayli, Cengiz, Canturk, Durmus, Akyol, and Menkse, 2005) (Hung, et al., 2005) Rhabdomylosis has been reported when colchicine was used with atorvastatin. (Tufan, Cavus, Altintas, Iskit, and Topeli, 2006)
When used for long periods of time, such as in the treatment of familial Meditarranean fever, colchicine can alter drug absorption and cause gastrointestinal effects such as lactose intolerance. (Ben-Chetrit and Levy, 2003)The available data suggest a dosage adjustment is necessary with strong CYP3A4 and P-gp inhibitors.
USE IN SPECIFIC POPULATIONS
Use in Pregnancy
Colchicine is an FDA Pregnancy Category C
Drug. There are no adequate and well-controlled studies with colchicine
in pregnant women. Colchicine crosses the human placenta. Published animal
reproduction and development studies indicate that colchicine causes embryofetal
toxicity, teratogenicity, and altered postnatal development at exposures within
or above the clinical therapeutic range. Colchicine Tablets should be used
during pregnancy only if the potential benefit justifies the potential risk to
the fetus.
Labor and Delivery
The effect of colchicine on labor and delivery is not known.
Colchicine is excreted into human milk. Limited information
suggests that exclusively breastfed infants receive less than 10 percent of the
maternal weight-adjusted dose. While there are no published reports of adverse
effects in breast-feeding infants of mothers taking colchicine, colchicine can
affect gastrointestinal cell renewal and permeability. Caution should be
exercised and breastfeeding infants should be observed for adverse effects when
colchicine is administered to a nursing woman.
Because of the increased incidence of decreased renal function in
the elderly population, and the higher incidence, in the elderly population, of
other co-morbid conditions, which often require the use of other medications, it
is essential to carefully consider the need for a reduction in the dosage of
Colchicine, when elderly patients are treated with Colchicine.
Please see WARNINGS AND PRECAUTIONS for discussion of usage in patients with Renal or Hepatic Dysfunction.
Use in Patients with Hepatic DysfunctionPlease see WARNINGS AND PRECAUTIONS for discussion of usage in patients with Renal or Hepatic Dysfunction.
DRUG ABUSE AND DEPENDENCE
Tolerance, abuse, or dependence from colchicine has not been reported.
OVERDOSAGE
The dose of colchicine that induces significant toxicity isnot known. Fatalities have been reported in patients after ingesting a dose as low as 7 mg over a 4-day period, while other patients have reportedly survived after ingesting more than 60 mg. A review of 150 patients who overdosed on colchicine found that those who ingested less than 0.5 mg/kg survived and tended to have milder adverse reactions, such as gastrointestinal symptoms, whereas those who ingested from 0.5 to 0.8 mg/kg had more severe adverse reactions, including myelosuppression. There was 100% mortality among patients who ingested more than 0.8 mg/kg.
- The first stage of acute colchicine toxicity typically begins within 24
hours of ingestion and includes gastrointestinal symptoms, such as abdominal
pain, nausea, vomiting, diarrhea, and significant fluid loss, leading to volume
depletion. Peripheral leukocytosis may also be seen.
- Life-threatening complications occur during the second stage, which occurs
24 to 72 hours after drug administration, attributed to multi-organ failure and
its associated consequences. Death usually results from respiratory depression
and cardiovascular collapse. If the patient survives, recovery of multi-organ
injury may be accompanied by rebound leukocytosis and alopecia starting about 1
week after the initial ingestion.
- Treatment of colchicine overdose should begin with gastric lavage and measures to prevent shock. Otherwise, treatment is symptomatic and supportive. No specific antidote is known. Colchicine is not effectively removed by dialysis.
DESCRIPTION
Colchicine is an alkaloid obtained from the plant Colchicum autumnale.
The chemical name for colchicine is (S)-N-(5,6,7,9- tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzol[α]heptalen-7-yl) acetamide. The structural formula is represented below:
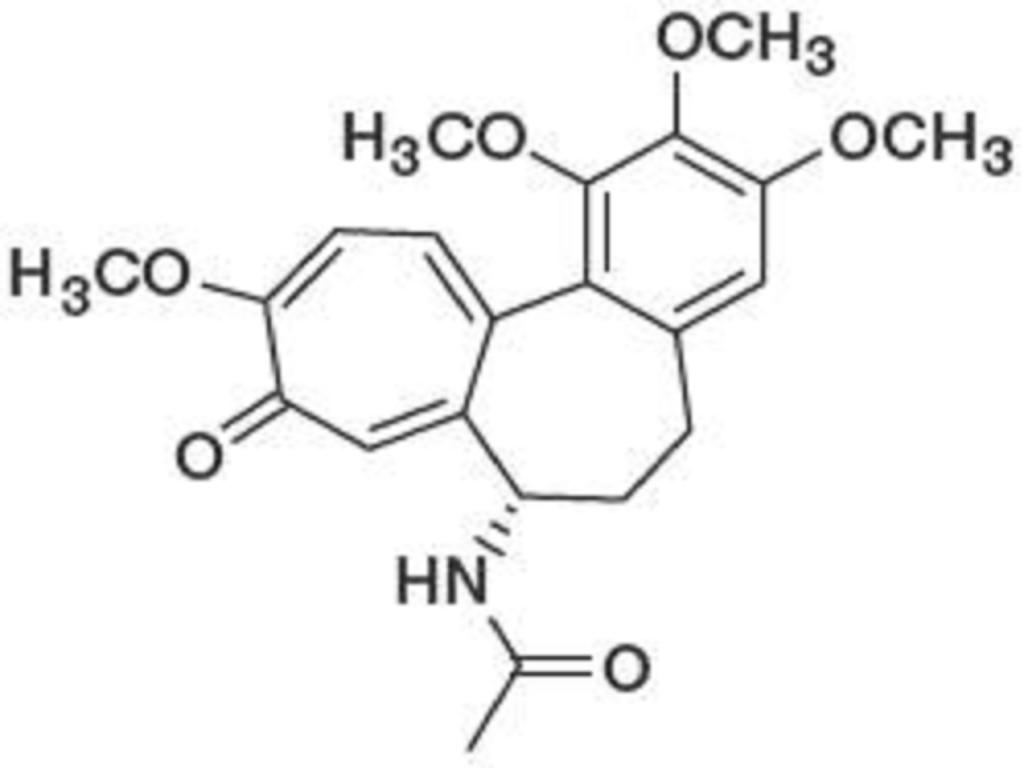
Colchicine consists of pale yellow scales or powder; it darkens on exposure to light. Colchicine is soluble in water, freely soluble in alcohol and in chloroform, and slightly soluble in ether.
Each tablet contains 0.6 mg (1/100 grain) colchicine USP and the following inactive ingredients: colloidal silicon dioxide, lactose anhydrous, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Mechanism of Action
Colchicine’s effectiveness as a treatment for gout has been postulated to be due to its ability to block neutrophil-mediated inflammatory responses induced by monosodium urate crystals in synovial fluid by disrupting the function of the neutrophil’s cytoskeleton and interfering with microtubule assembly. This results in the prevention of activation, degranulation, and migration of neutrophils to sites of inflammation. New evidence suggests that colchicine may interfere with the intracellular assembly of the inflammasome complex found in neutrophils and monocytes that mediate IL-1b activation.
Colchicine is predominantly eliminated by biliary excretion and through the stool; gastrointestinal tract lining cell turnover has a variable role in colchicine elimination. Colchicine is extruded from cells, including the enteric lining cells, into the gastrointestinal tract, mediated by the multidrug resistance transporter molecule ABCB1 (full name: ATP-binding cassette subfamily B member, 1, MDRI, PGYI; also known as P-glycoprotein [P-gp] or CD243). Normally, a lesser but significant role in colchicine metabolism (-5 to 20%) is played by enteric and hepatic cytochrome P450 3E4 (CYP3A4), which catalyzes demethylation of colchicine to inactive metabolites. Hepatic demethylation of colchicine dependent on CYP3A4 occurs before hepatobiliary excretion of colchicine.
Renal elimination has been estimated to be responsible for 10 to 20% of drug disposition in normal subjects. CYP3A4 and renal disposition of colchicine become more critical with certain drug-drug interactions that affect ABCB 1, with hepatobiliary dysfunction and with aging.
Colchicine is not removed by hemodialysis.
PharmacokineticsAbsorption
In healthy adults, colchicine appears to be readily absorbed when orally administered. When Colchicine Tablets 0.6 mg were administered to 18 healthy volunteers, a Cmax of about 2 ng/Ml at a tmax of 1 hou was observed.
Distribution
Colchicine is lipid-soluble and has a mean apparent volume of distribution in healthy young volunteers of approximately 5 to 8 L/kg. Colchicine binding to serum protein is 39% (plus or minus 5%), and binds primarily to albumin; it crosses the placenta and distributes into breast milk.
Metabolism
There are two primary metabolites, 2-O-demethylcolchicine and 3-O-demethylcolchicine (known as 2- and 3-DMC, respectively), and one minor metabolite, 10-O-demethylcolchicine (also known as 10-DMC or colchiceine). Human liver microsomes studies have shown that CYP3A4 is involved in the metabolism of colchicine to 2- and 3-DMC. In vivo, exposure to 2-DMC and 3-DMC metabolites is less than 5% of parent drug.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of colchicine have not been evaluated. Due to the potential for colchicine to produce aneuploid cells (cells with an unequal number of chromosomes), colchicine presents a theoretical increased risk of malignancy.
Mutagenesis
Colchicine was negative for mutagenicity in the bacterial reverse mutation assay. In a chromosomal aberration assay in cultured human white blood cells, colchicine treatment resulted in the formation of micronuclei. Since published studies demonstrated that colchicine induces aneuploidy from the process of mitotic nondisjunction without structural DNA changes, colchicine is not considered clastogenic, although micronuclei are formed.
Impairment of Fertility
Published nonclinical studies demonstrated that colchicine-induced disruption of microtubule formation affects meiosis and mitosis. Reproductive studies also reported abnormal sperm morphology and reduced sperm counts in males, and interference with sperm penetration, second meiotic division, and normal cleavage in females when exposed to colchicine. Colchicine administered to pregnant animals resulted in fetal death and teratogenicity. These effects were dose dependent, with the timing of exposure critical for the effects on embryofetal development. The nonclinical doses evaluated were generally higher than an equivalent human therapeutic dose, but safety margins for reproductive and developmental toxicity could not be determined. There are published studies that show that men and women taking colchicine can safely have children.
Case reports and epidemiology studies in human male subjects on colchicine therapy indicated that infertility from colchicine is rare. A case report indicated that azoospermia was reversed when therapy was stopped. Case reports and epidemiology studies in female subjects on colchicine therapy have not established a clear relationship between colchicine use and female infertility.
REFERENCES
Akdag, I., Erosy, A., Kahvecioglu, S., Gullulu, M., and Dilek, K. (2006). Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure. Journal of Nephrology , 19, 515-517.
Alayli, G., Cengiz, K., Canturk, F., Durmus, D., Akyol, Y., and Menkse, E. (2005). Acute Myopathy in a Patient with Concomitant Use of Pravastatin and Colchicine. The Annals of Pharmacology , 39, 1358-1361.
Ben-Chetrit, D., and Levy, M. (2003). Reproductive system in familial Mediterranean fever: an overview. Annals of Rheumatic Diseases , 62, 916-919.
Holtzman, C., Wiggins, B., and Spinler, S. (2006). Role of P-glycoprotein in Statin Drug Interactions. Pharmacotherapy , 26 (11), 1601-1607.
Hsu, W.-C., Chen, W.-H., Chang, M.-T.,and Chiu, H.-C. (2002). Colchicine-Induced Acute Myopathy in a Patient with Concomitant Use of Simvastatin. Clinical Neuropharmacology , 25 (5), 266-268.
Hung, I., Wu, A. C., Tang, B., To, K., Yeung, C., Woo, P., et al. (2005). Fatal Interaction between Clarithromycin and Colchicine in Patients with Renal Insufficiency; A Retrospective Study. Clinical Infectious Diseases , 41, 291-300.
Niel, E., and Scherrmann, J.-M. (2006). Colchicine today. Joint Bone Spine , 73, 672-678.
Rollot, F., Pajot, O., Chauvelot-Moachon, L., Nazal, E., Kelaidi, C., andBlanche, P. (2004). Acute Colchicine Intoxication During Clarithromycin Administration. The Annals of Pharmacotherapy , 38, 2074-2077.
Tufan, A. D., Cavus, S., Altintas, N., Iskit, A., and Topeli, A. (2006). Rhabdomyolysis in a Patient Treated with Colchicine and Atorvastatin. The Annals of Pharmacotherapy , 40, 1466-1469.
HOW SUPPLIED
Colchicine Tablets, USP 0.6 mg are white, round, unscored, shallow concave compressed tablets with WEST-WARD 201on the tablets.
| Bottles of 10 | NDC 54868-0998-5 |
| Bottles of 20 | NDC 54868-0998-6 |
| Bottels of 25 | NDC 54868-0998-4 |
| Bottles of 30 | NDC 54868-0998-0 |
| Blister pack of 30 | NDC 54868-0998-2 |
| Bottles of 60 | NDC 54868-0998-3 |
| Bottles of 90 | NDC 54868-0998-7 |
| Bottles of 100 | NDC 54868-0998-1 |
STORAGE AND HANDLING
Store at 20° to 25° C (68° to 77° F).
[See USP Controlled Room Temperature]
Protect from light.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

| COLCHICINE
colchicine tablet |
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 05/12/1995 | 12/29/2010 | |
| Labeler - Physicians Total Care, Inc. (194123980) |