ALLERX DOSE PACK
-
pseudoephedrine hydrochloride, phenylephrine hydrochloride, chlorpheniramine maleate, methscopolamine nitrate
Cornerstone Therapeutics Inc.
----------
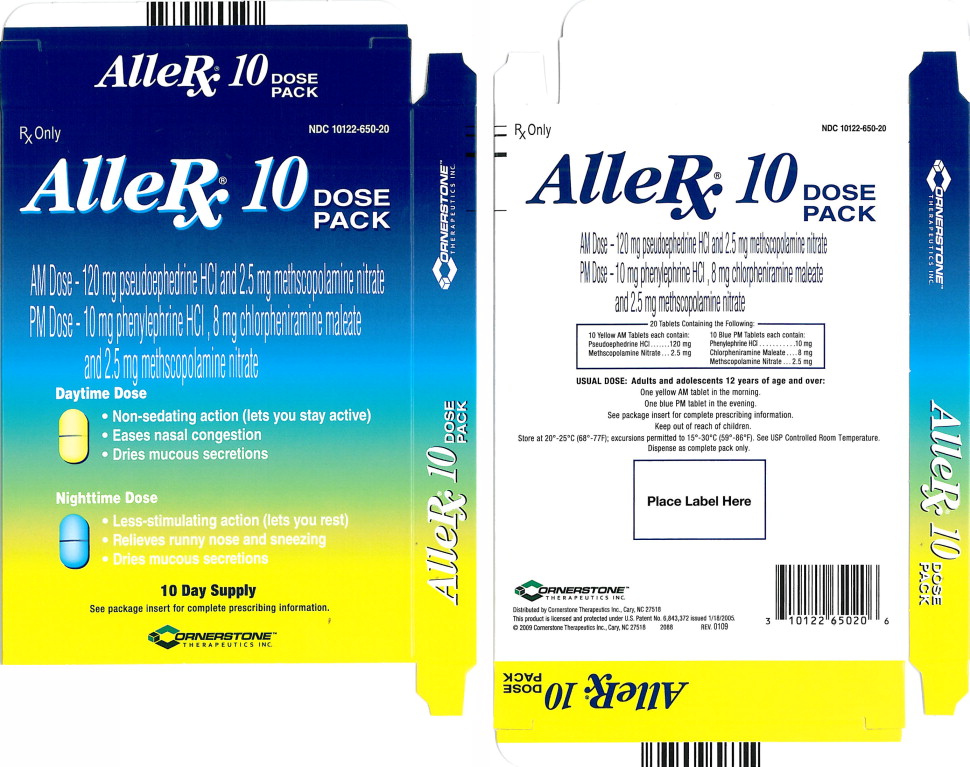
AlleRx®10 DOSE PACK
AM Dose - 120 mg pseudoephedrine HCI and 2.5 mg methscopolamine nitrate
PM Dose - 10 mg phenylephrine HCI, 8 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
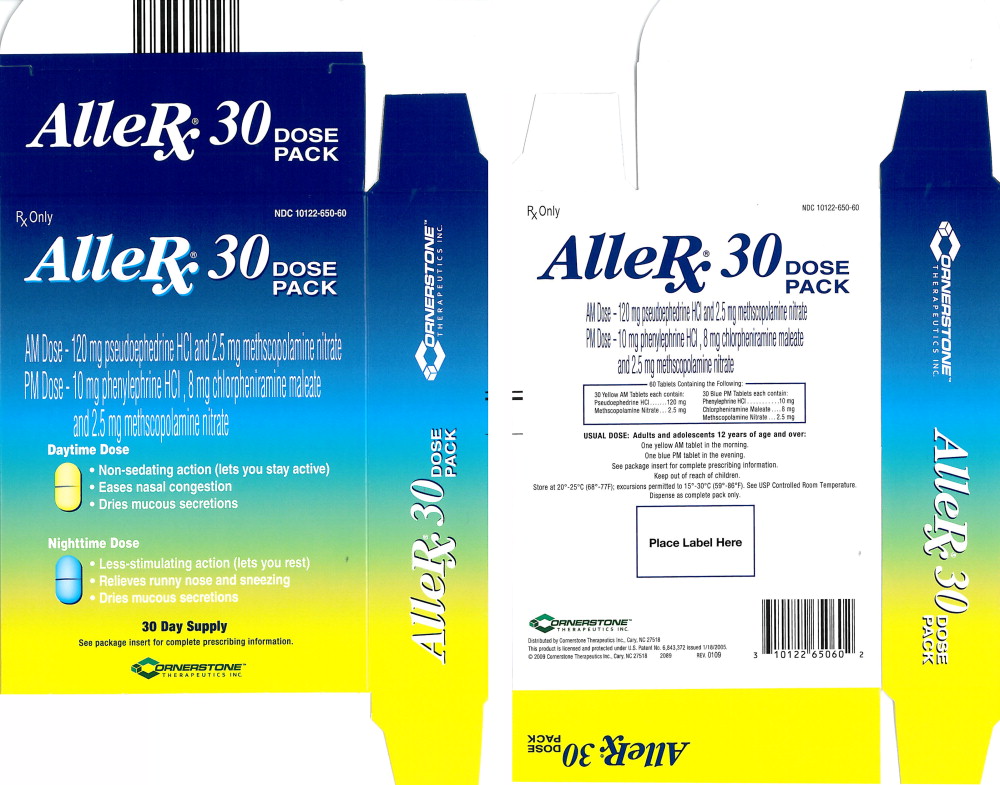
AlleRx®30 DOSE PACK
AM Dose - 120 mg pseudoephedrine HCI and 2.5 mg methscopolamine nitrate
PM Dose - 10 mg phenylephrine HCI, 8 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
DESCRIPTION
| Each AM tablet contains: | Each PM tablet contains: |
| Pseudoephedrine HCl ................... 120 mg | Phenylephrine HCl.........................10 mg |
| Methscopolamine Nitrate ............... 2.5 mg | Chlorpheniramine Maleate............8 mg |
| Methscopolamine Nitrate...............2.5 mg |
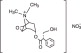
Chlorpheniramine maleate is an antihistamine having the chemical name 2-pyridinepropanamine, gamma-(4 chlorophenyl)-N, N-dimethyl-, (Z)-2-butenedioate (1:1).
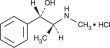
Pseudoephedrine hydrochloride is a nasal decongestant. Chemically it is [S-(R*,R*)]-α-[1(methylamino) ethyl]-benzenemethanol hydrochloride; C10H15NO•HCl, MW = 201.7.
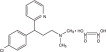
Methscopolamine nitrate is an anticholinergic having the chemical name 3-oxa-9-azoniatricyclo [3.3.1.0 2 4] nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9, 9-dimethyl-, nitrate, [7(S)-(1α, 2β, 4β, 5α, 7β)]; C17H21NO4•CH3NO3, MW = 80.4.
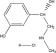
Phenylephrine hydrochloride is a decongestant having the chemical name (-)-m-Hydroxy-α-[(methyl amino)methyl] benzyl alcohol hydrochloride.
Inactive Ingredients:
AM tablets: Each yellow AM tablet contains Dibasic Calcium Phosphate, D&C Yellow #10 (aluminum lake) Dye, Ethylcellulose, Magnesium Stearate and Stearic Acid.
PM tablets: Each blue PM tablet contains Hypromellose, Dicalcium Phosphate, Talc, FD&C Blue # 1, Stearic Acid and Magnesium Stearate.
CLINICAL PHARMACOLOGY
Pseudoephedrine HCl is an indirect-acting sympathomimetic amine that exerts a decongestant action on the nasal mucosa. It does this by vasoconstriction, which results in reduction of tissue hyperemia, edema, nasal congestion, and an increase in nasal airway patency. In the usual dose it has minimal vasopressor effects. Pseudoephedrine HCl is rapidly and almost completely absorbed from the gastrointestinal tract. It has a plasma half-life of 6 to 8 hours. Alkaline urine is associated with slower elimination of the drug. The drug is distributed to the body tissues and fluids, including the central nervous system (CNS). Approximately 50% to 75% of the administered dose is excreted unchanged in the urine; the metabolized in the liver to inactive compounds by N-demethylation, parahydroxylation, and oxidative deamination.
Chlorpheniramine maleate is an alkylamine-type antihistamine. This group of antihistamines is among the most active histamine antagonists and is generally effective in relatively low doses.
Methscopolamine nitrate is a quaternary ammonium derivative of the anticholinergic scopolamine which possesses the peripheral actions of the belladonna alkaloids, but does not exhibit the central actions because of its lack of ability to cross the blood-brain barrier. Its antimuscarinic effect causes inhibition of salivary secretions, reduction in volume and total acid content of gastric secretion, and inhibition of gastrointestinal motility. It is poorly and unreliably absorbed. Drug effects appear in about one hour and persist for about 4 to 6 hours. It is excreted primarily in the urine and bile, or as unabsorbed drug in feces.
Phenylephrine HCl is a sympathomimetic amine which acts on the alpha adrenergic receptors. Clinically, phenylephrine shrinks swollen mucous membranes, reduces tissue hyperemia, edema, and nasal congestion, and increases nasal airway patency.
INDICATIONS AND USAGE
For the temporary relief of symptoms associated with allergic rhinitis.
CONTRAINDICATIONS
This product is contraindicated in patients with hypersensitivity to pseudoephedrine HCl, methscopolamine nitrate, phenylephrine HCl, and chlorpheniramine maleate. AlleRx® is contraindicated in patients with severe hypertension, severe coronary artery disease, patients on monoamine oxidase inhibitor (MAOI) therapy or within 14 days of stopping monoamine oxidase inhibitor (MAOI) therapy, and in nursing mothers. AlleRx is also contraindicated in patients with narrow-angle glaucoma, urinary retention, peptic ulcer, and during an asthmatic attack.
WARNINGS
Sympathomimetic amines should be used cautiously in patients with hypertension, diabetes mellitus, ischemic heart disease, hyperthyroidism, increased intraocular pressure, and prostatic hypertrophy. Sympathomimetics may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension. The elderly (60 years or older) are more likely to exhibit adverse reactions. Antihistamines may cause excitability, especially in children. At dosages higher than the recommended dose, nervousness, dizziness, or sleeplessness may occur. Do not exceed recommended dosage.
Hypertensive crisis can occur with concurrent use of pseudoephedrine HCl and MAOI, and for 14 days after stopping therapy. Methscopolamine nitrate may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or performing hazardous work while taking AlleRx.
Co-administration of sildenafil citrate and other organic nitrates has been shown to potentiate the hypotension effects of nitrates. Co-administration of AlleRx and sildenafil citrate has not been studied. Therefore, the use of sildenafil citrate and AlleRx together is not recommended.
PRECAUTIONS
General: AlleRx should be used with caution in patients with diabetes mellitus, hypertension, cardiovascular disease, and hyperactivity to sympathomimetic amines. Antihistamines may cause drowsiness, and ambulatory patients who operate machinery or motor vehicles should be cautioned accordingly. Methscopolamine nitrate should be used with caution in the elderly and all patients with autonomic neuropathy, hepatic or renal disease, or ulcerative colitis.
Drug Interactions: Do not prescribe AlleRx for use in patients that are now taking prescription MAOIs (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 14 days after stopping MAOI drug therapy. Beta-adrenergic blockers and MAOIs may potentiate the pressor effect of pseudoephedrine HCl. Concurrent use of digitalis glycosides may increase the possibility of cardiac arrhythmias. Sympathomimetics may reduce the hypotensive effects of guanethidine, mecamylamine, methyldopa, reserpine and veratrum alkaloids. Concurrent use of pseudoephedrine HCl with other sympathomimetic amines may increase pressor or cardiovascular effects of either medication.
The use of sympathomimetic amines may result in additive CNS depressant effects when coadministered with alcohol, antihistamines, psychotropics, or other drugs which produce CNS depression. Concurrent use of tricyclic antidepressants may antagonize the effects sympathomimetic amines.
Additive anticholinergic effects may result from concomitant use with antipsychotics, tricyclic antidepressants, and other drugs with anticholinergic effects. Concomitant administration with antacids may interfere with the absorption of methscopolamine nitrate.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Animal studies to assess the long-term carcinogenic and mutagenic potential or the effect on fertility in animals or humans have not been performed.
Pregnancy Category C: It is not known whether AlleRx can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. AlleRx should be given to a pregnant woman only if clearly needed.
Nursing Mothers: It is not known whether this combination drug is excreted in human milk. However, pseudoephedrine HCl and phenylephrine HCl administered alone distributes into the breast milk of lactating human females; therefore, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: The safety and effectiveness in children under 12 years of age have not been established.
Geriatric Use: The elderly (60 years and older) are more likely to experience adverse reactions to sympathomimetics. Overdosage of sympathomimetics in this age group may cause hallucinations, convulsions, CNS depression, and/or death. Sympathomimetic amines are known to be substantially excreted by the kidneys, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
ADVERSE REACTIONS
Adverse reactions include drowsiness, lassitude, nausea, giddiness, dryness of mouth, blurred vision, cardiac palpitations, flushing, and increased irritability or excitement (especially in children). Some individuals may display sympathomimetic amine effects such as tachycardia, palpitations, headache, dizziness, or nausea. Sympathomimetics have been associated with certain untoward reactions including fear, anxiety, nervousness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension. Urinary retention may occur in patients with prostatic hypertrophy. Antihistamines and anticholinergics may cause drowsiness, dizziness, blurred vision, and excessive dryness of the nose, throat, and mouth.
DRUG ABUSE AND DEPENDENCE
Rebound congestion may occur after vasoconstriction subsides when pseudoephedrine HCl is discontinued. Patients may increase the amount of drug and frequency of use, producing toxicity and perpetuating the rebound congestion. Excessive use may cause systemic effects which are more likely in the elderly. Habituation and toxic psychosis have followed long-term high-dose therapy.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE
The treatment of overdosage should provide symptomatic and supportive care. Induction of emesis and gastric lavage may be performed if the patient is alert and seen within early hours after ingestion. Drug remaining in the stomach may be absorbed by the administration of activated charcoal. Stimulants should not be used because they may precipitate convulsions. If convulsions or marked CNS excitement occurs, treatment with appropriate measures is indicated. Since the effects of AlleRx last up to 12 hours, the patient should be monitored for at least that length of time and treated as necessary.
DOSAGE AND ADMINISTRATION
Adults and adolescents 12 years of age and over: One yellow AM tablet in the morning and one blue PM tablet in the evening. AlleRx is not recommended for children under 12 years of age.
HOW SUPPLIED
(NDC 10122-650-20) AlleRx 10 Day Dose Pack Treatment Regimen, containing 20 tablets as follows:
10 yellow, elongated and scored AM tablets debossed with “CBP” on one side and “01” to the right of the score on the other side, each containing 120 mg of pseudoephedrine HCl and 2.5 mg of methscopolamine nitrate.
10 blue, elongated and scored PM tablets debossed with “CBP” on one side and “05” to the right of the score on the other side, each containing 10 mg phenylephrine HCl, 8 mg of chlorpheniramine maleate and 2.5 mg of methscopolamine nitrate.
(NDC 10122-650-60) AlleRx 30 Day Dose Pack Treatment Regimen, containing 60 tablets as follows:
30 yellow, elongated and scored AM tablets debossed with “CBP” on one side and “01” to the right of the score on the other side, each containing 120 mg of pseudoephedrine HCl and 2.5 mg of methscopolamine nitrate.
30 blue, elongated and scored PM tablets debossed with “CBP” on one side and “05” to the right of the score on the other side, each containing 10 mg phenylephrine HCl, 8 mg of chlorpheniramine maleate and 2.5 mg of methscopolamine nitrate.
(NDC 10122-650-02) AlleRx Tablets 2-pack sample containing 1 yellow AM tablet and 1 blue PM tablet per sample.
Keep out of reach of pediatric population.
Store at 20° - 25° C (68° - 77° F); excursions permitted to 15° - 30° C (59° - 86° F). See USP Controlled Room Temperature.
Distributed by Cornerstone Therapeutics Inc., Cary, NC 27518.
This product is licensed and protected under U.S. Patent No. 6,843,372, issued 01/18/2005.
Rx Only
CORNERSTONE
THERAPEUTICS INC.™
© 2009 Cornerstone Therapeutics Inc., Cary, NC 27518
CTA740C0109
| ALLERX
DOSE PACK
pseudoephedrine hydrochloride, phenylephrine hydrochloride, chlorpheniramine maleate, methscopolamine nitrate kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 02/01/2008 | ||
| Labeler - Cornerstone Therapeutics Inc. (153886994) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sovereign Pharmaceuticals, Ltd. | 623168267 | MANUFACTURE | |