fareston (toremifene citrate) tablet
[GTx, Inc.]
1E Rev 12/2004
DESCRIPTION
FARESTON (toremifene citrate) Tablets for oral administration each contain 88.5 mg of toremifene citrate, which is equivalent to 60 mg toremifene.
FARESTON is a nonsteroidal antiestrogen. The chemical name of toremifene is: 2-{p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy}-N,N-dimethylethylamine citrate (1:1). The structural formula is:
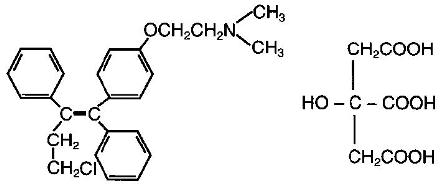
and the molecular formula is C26H28CINO • C6H8O7. The molecular weight of toremifene citrate is 598.10. The pKa is 8.0. Water solubility at 37˚C is 0.63 mg/mL and in 0.02N HCI at 37˚C is 0.38 mg/mL.
FARESTON is available only as tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch.
CLINICAL PHARMACOLOGY
Mechanism of Action: Toremifene is a nonsteroidal triphenylethylene derivative. Toremifene binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities, depending upon the duration of treatment, animal species, gender, target organ, or endpoint selected. In general, however, nonsteroidal triphenylethylene derivatives are predominantly antiestrogenic in rats and humans and estrogenic in mice. In rats, toremifene causes regression of established dimethylbenzanthracene (DMBA)-induced mam-mary tumors. The antitumor effect of toremifene in breast cancer is believed to be mainly due to its antiestrogenic effects, ie, its ability to compete with estrogen for binding sites in the cancer, blocking the growth-stimulating effects of estrogen in the tumor.
Toremifene causes a decrease in the estradiol-induced vaginal cornification index in some postmenopausal women, indicative of its antiestrogenic activity. Toremifene also has estrogenic activity as shown by decreases in serum gonadotropin concentrations (FSH and LH).
Pharmacokinetics: The plasma concentration time profile of toremifene declines biexponentially after absorption with a mean distribution half-life of about 4 hours and an elimination half-life of about 5 days. Elimination half-lives of major metabolites, N-demethyltoremifene and (deaminohydroxy) toremifene were 6 and 4 days, respectively. Mean total clearance of toremifene was approximately 5L/h.
Absorption and Distribution: Toremifene is well absorbed after oral administration and absorption is not influenced by food. Peak plasma concentrations are obtained within 3 hours. Toremifene displays linear pharmacokinetics after single oral doses of 10 to 680 mg. After multiple dosing, dose proportionality was observed for doses of 10 to 400 mg. Steady-state concentrations were reached in about 4-6 weeks. Toremifene has an apparent volume of distribution of 580 L and binds extensively (>99.5%) to serum proteins, mainly to albumin.
Metabolism and Excretion: Toremifene is extensively metabolized, principally by CYP3A4 to N-demethyltoremifene, which is also antiestrogenic but with weak in vivo antitumor potency. Serum concentrations of N-demethyltoremifene are 2 to 4 times higher than toremifene at steady state. Toremifene is eliminated as metabolites predominantly in the feces, with about 10% excreted in the urine during a 1-week period. Elimination of toremifene is slow, in part because of enterohepatic circulation.
Special Populations:
Renal insufficiency: The pharmacokinetics of toremifene and N-demethyltoremifene were similar in normals and in patients with impaired kidney function.
Hepatic insufficiency: The mean elimination half-life of toremifene was increased by less than twofold in 10 patients with hepatic impairment (cirrhosis or fibrosis) compared to subjects with normal hepatic function. The pharmacokinetics of N-demethyltoremifene were unchanged in these patients. Ten patients on anticonvulsants (phenobarbital, clonazepam, phenytoin, and carbamazepine) showed a twofold increase in clearance and a decrease in the elimination half-life of toremifene.
Geriatric patients: The pharmacokinetics of toremifene were studied in 10 healthy young males and 10 elderly females following a single 120 mg dose under fasting conditions. Increases in the elimination half-life (4.2 versus 7.2 days) and the volume of distribution (457 versus 627 L) of toremifene were seen in the elderly females without any change in clearance or AUC.
Race: The pharmacokinetics of toremifene in patients of different races has not been studied.
Drug-drug interactions: No formal drug-drug interaction studies with toremifene have been performed.
CLINICAL STUDIES
Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of FARESTON for the treatment of breast cancer in postmenopausal women. The patients were randomized to parallel groups receiving FARESTON 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively. The studies included postmenopausal patients with estrogen-receptor (ER) positive or estrogen-receptor (ER) unknown metastatic breast cancer. The patients had at least one measurable or evaluable lesion. The primary efficacy variables were response rate (RR) and time to progression (TTP). Survival (S) was also determined. Ninety-five percent confidence intervals (95% CI) were calculated for the difference in RR between FAR60 and TAM groups and the hazard ratio (relative risk for an unfavorable event, such as disease progression or death) between TAM and FAR60 for TTP and S.
Two of the 3 studies showed similar results for all effectiveness endpoints. However, the Nordic Study showed a longer time to progression for tamoxifen (see table).
| Study | North American | Eastern European | Nordic | |||
|---|---|---|---|---|---|---|
|
1CR = complete response; 2PR = partial response; 3RR = response rate; 4CI = confidence interval |
||||||
| Treatment Group | FAR60 | TAM20 | FAR60 | TAM40 | FAR60 | TAM40 |
| No. Patients | 221 | 215 | 157 | 149 | 214 | 201 |
| Responses | ||||||
| CR1 + PR2 | 14+33 | 11+30 | 7+25 | 3+28 | 19+48 | 19+56 |
| RR3 (CR + PR)% | 21.3 | 19.1 | 20.4 | 20.8 | 31.3 | 37.3 |
| Difference in RR 95% CI4 for | 2.2 | -0.4 | -6.0 | |||
| Difference in RR | -5.8 to 10.2 | -9.5 to 8.6 | -15.1 to 3.1 | |||
| Time to Progression (TTP) | ||||||
| Median TTP (mo.) | 5.6 | 5.8 | 4.9 | 5.0 | 7.3 | 10.2 |
| Hazard Ratio (TAM/FAR) 95% CI4 for | 1.01 | 1.02 | 0.80 | |||
| Hazard Ratio (%) | 0.81 to 1.26 | 0.79 to 1.31 | 0.64 to 1.00 | |||
| Survival (S) | ||||||
| Median S (mo.) | 33.6 | 34.0 | 25.4 | 23.4 | 33.0 | 38.7 |
| Hazard Ratio (TAM/FAR) 95% CI4 for | 0.94 | 0.96 | 0.94 | |||
| Hazard Ratio (%) | 0.74 to 1.24 | 0.72 to 1.28 | 0.73 to 1.22 | |||
The high-dose groups, toremifene 200 mg daily in the North American Study and 240 mg daily in the Eastern European Study, were not superior to the lower toremifene dose groups, with response rates of 22.6% AND 28.7%, median times to progression of 5.6 and 6.1 months, and median survivals of 30.1 and 23.8 months, respectively. The median treatment duration in the three pivotal studies was 5 months (range 4.2-6.3 months).
INDICATION AND USAGE
FARESTON is indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors.
CONTRAINDICATIONS
FARESTON is contraindicated in patients with known hypersensitivity to the drug.
WARNINGS
Hypercalcemia and Tumor Flare: As with other antiestrogens, hypercalcemia and tumor flare have been reported in some breast cancer patients with bone metastases during the first weeks of treatment with FARESTON. Tumor flare is a syndrome of diffuse musculoskeletal pain and erythema with increased size of tumor lesions that later regress. It is often accompanied by hypercalcemia. Tumor flare does not imply failure of treatment or represent tumor progression. If hypercalcemia occurs, appropriate measures should be instituted and if hypercalcemia is severe, FARESTON treatment should be discontinued.
Tumorigenicity: Since most toremifene trials have been conducted in patients with metastatic disease, adequate data on the potential endometrial tumorigenicity of long-term treatment with FARESTON are not available. Endometrial hyperplasia has been reported. Some patients treated with FARESTON have developed endometrial cancer, but circumstances (short duration of treatment or prior antiestrogen treatment or premalignant conditions) make it difficult to establish the role of FARESTON.
Endometrial hyperplasia of the uterus was observed in monkeys following 52 weeks of treatment at ≥1 mg/kg and in dogs following 16 weeks of treatment at ≥3 mg/kg with toremifene (about 1/4 and 1.4 times, respectively, the daily maximum recommended human dose on a mg/m2 basis).
Pregnancy: FARESTON may cause fetal harm when administered to pregnant women. Studies in rats at doses ≥1.0 mg/kg/day (about 1/4 the daily maximum recommended human dose on a mg/m2 basis) administered during the period of organogenesis, have shown that toremifene is embryotoxic and fetotoxic, as indicated by intrauterine mortality, increased resorption, reduced fetal weight, and fetal anomalies; including malformation of limbs, incomplete ossification, misshapen bones, ribs/spine anomalies, hydroureter, hydronephrosis, testicular displacement, and subcutaneous edema. Fetal anomalies may have been a consequence of maternal toxicity. Toremifene has been shown to cross the placenta and accumulate in the rodent fetus.
In rodent models of fetal reproductive tract development, toremifene produced inhibition of uterine development in female pups similar to diethylstilbestrol (DES) and tamoxifen. The clinical relevance of these changes is not known.
Embryotoxicity and fetotoxicity were observed in rabbits at doses ≥1.25 mg/kg/day and 2.5 mg/kg/day, respectively (about 1/3 and 2/3 the daily maximum recommended human dose on a mg/m2 basis); fetal anomalies included incomplete ossification and anencephaly.
There are no studies in pregnant women. If FARESTON is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus or potential risk for loss of the pregnancy.
PRECAUTIONS
General: Patients with a history of thromboembolic diseases should generally not be treated with FARESTON. In general, patients with preexisting endometrial hyperplasia should not be given long-term FARESTON treatment. Patients with bone metastases should be monitored closely for hypercalcemia during the first weeks of treatment (see WARNINGS). Leukopenia and thrombocytopenia have been reported rarely; leukocyte and platelet counts should be monitored when using FARESTON in patients with leukopenia and thrombocytopenia.
Information for Patients: Vaginal bleeding has been reported in patients using FARESTON. Patients should be informed about this and instructed to contact their physician if such bleeding occurs.
Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia and instructed to contact their physician for further assessment if such signs or symptoms occur.
Laboratory Tests: Periodic complete blood counts, calcium levels, and liver function tests should be obtained.
Drug-drug Interactions: Drugs that decrease renal calcium excretion, eg, thiazide diuretics, may increase the risk of hypercalcemia in patients receiving FARESTON. There is a known interaction between antiestrogenic compounds of the triphenylethylene derivative class and coumarin-type anticoagulants (eg, warfarin), leading to an increased prothrombin time. When concomitant use of anticoagulants with FARESTON is necessary, careful monitoring of the prothrombin time is recommended.
Cytochrome P450 3A4 enzyme inducers, such as phenobarbital, phenytoin, and carbamazepine increase the rate of toremifene metabolism, lowering the steady-state concentration in serum. Metabolism of toremifene may be inhibited by drugs known to inhibit the CYP3A4-6 enzymes. Examples of such drugs are ketoconazole and similar antimycotics as well as erythromycin and similar macrolides. This interaction has not been studied and its clinical relevance is uncertain.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Conventional carcinogenesis studies in rats at doses of 0.12 to 12 mg/kg/day (about 1/100 to 1.5 times the daily maximum recommended human dose on a mg/m2 basis) for up to 2 years did not show evidence of carcinogenicity. Studies in mice at doses of 1.0 to 30.0 mg/kg/day (about 1/15 to 2 times the daily maximum recommended human dose on a mg/m2 basis) for up to 2 years revealed increased incidence of ovarian and testicular tumors, and increased incidence of osteoma and osteosarcoma. The significance of the mouse findings is uncertain because of the different role of estrogens in mice and the estrogenic effect of toremifene in mice. An increased incidence of ovarian and testicular tumors in mice has also been observed with other human antiestrogenic agents that have primarily estrogenic activity in mice.
Toremifene has not been shown to be mutagenic in in vitro tests (Ames and E. coli bacterial tests). Toremifene is clastogenic in vitro (chromosomal aberrations and micronuclei formation in human lymphoblastoid MCL-5 cells) and in vivo (chromosomal aberrations in rat hepatocytes). No significant adduct formation could be detected using 32P post-labeling in liver DNA from rats administered toremifene when compared to tamoxifen at similar doses. A study in cultured human lymphocytes indicated that adducting activity of toremifene, detected by 32P post-labeling, was about 1/6 that of tamoxifen at approximately equipotent concentrations. In addition, the DNA adducting activity of toremifene in salmon sperm, using 32P post-labeling, was 1/6 and 1/4 that observed with tamoxifen at equivalent concentrations following activation by rat and human microsomal systems, respectively. However, toremifene exposure is fourfold the exposure of tamoxifen based on human AUC in serum at recommended clinical doses.
Toremifene produced impairment of fertility and conception in male and female rats at doses ≥25.0 and 0.14 mg/kg/day, respectively (about 3.5 times and 1/50 the daily maximum recommended human dose on a mg/m2 basis). At these doses, sperm counts, fertility index, and conception rate were reduced in males with atrophy of seminal vesicles and prostate. In females, fertility and reproductive indices were markedly reduced with increased pre- and post-implantation loss. In addition, offspring of treated rats exhibited depressed reproductive indices. Toremifene produced ovarian atrophy in dogs administered doses ≥3 mg/kg/day (about 1.5 times the daily maximum recommended human dose on a mg/m2 basis) for 16 weeks. Cystic ovaries and reduction in endometrial stromal cellularity were observed in monkeys at doses ≥1 mg/kg/day (about 1/4 the daily maximum recommended human dose on a mg/m2 basis) for 52 weeks.
Pregnancy:Pregnancy Category D: (see WARNINGS).
Nursing mothers: Toremifene has been shown to be excreted in the milk of lactating rats. It is not known if this drug is excreted in human milk. (See WARNINGS and PRECAUTIONS).
Pediatric use: There is no indication for use of FARESTON in pediatric patients.
Geriatric use: The median ages in the three controlled studies ranged from 60 to 66 years. No significant age-related differences in FARESTON effectiveness or safety were noted.
Race: Fourteen percent of patients in the North American Study were non-Caucasian. No significant race-related differences in FARESTON effectiveness or safety were noted.
ADVERSE REACTIONS
Adverse drug reactions are principally due to the antiestrogenic hor-monal actions of FARESTON and typically occur at the beginning of treatment.
The incidences of the following eight clinical toxicities were prospectively assessed in the North American Study. The incidence reflects the toxicities that were considered by the investigator to be drug related or possibly drug related.
| North American Study | ||
| FAR60 | TAM20 | |
| n = 221 | n = 215 | |
| Hot Flashes | 35% | 30% |
| Sweating | 20% | 17% |
| Nausea | 14% | 15% |
| Vaginal Discharge | 13% | 16% |
| Dizziness | 9% | 7% |
| Edema | 5% | 5% |
| Vomiting | 4% | 2% |
| Vaginal Bleeding | 2% | 4% |
Approximately 1% of patients receiving FARESTON (n = 592) in the three controlled studies discontinued treatment as a result of adverse events (nausea and vomiting, fatigue, thrombophlebitis, depression, lethargy, anorexia, ischemic attack, arthritis, pulmonary embolism, and myocardial infarction).
Serious adverse events occurring in patients receiving FARESTON in the three major trials are listed in the table below.
|
* Most of the ocular abnormalities were observed in the North American Study in which on-study and biannual opthalmic examinations were performed. No cases of retinopathy were observed in any arm. |
||||||||||||
|
** Elevated defined as follows: North American Study: SGOT >100 IU/L; alkaline phosphatase >200 IU/L; bilirubin > 2 mg/dL. Eastern European and Nordic studies: SGOT, alkaline phosphatase, and bilirubin – WHO Grade 1 (1.25 times the upper limit of normal). |
||||||||||||
| Adverse Events | North American | Eastern European | Nordic | |||||||||
| FAR60 n=221(%) | TAM20 n=215(%) | FAR60 n=157(%) | TAM40 n=149(%) | FAR60 n=214(%) | TAM40 n=201(%) |
|||||||
| Cardiac | ||||||||||||
| Cardiac Failure | 2 | (1) | 1 | (<1) | - | 1 | (<1) | 2 | (1) | 3 | (1.5) | |
| Myocardial Infarction | 2 | (1) | 3 | (1.5) | 1 | (<1) | 2 | (1) | - | 1 | (<1) | |
| Arrhythmia | - | - | - | - | 3 | (1.5) | 1 | (<1) | ||||
| Angina Pectoris | - | - | 1 | (<1) | - | 1 | (<1) | 2 | (1) | |||
| Ocular* | ||||||||||||
| Cataracts | 22 | (10) | 16 | (7.5) | - | - | - | 5 | (3) | |||
| Dry Eyes | 20 | (9) | 16 | (7.5) | - | - | - | - | ||||
| Abnormal Visual Fields | 8 | (4) | 10 | (5) | - | - | - | 1 | (<1) | |||
| Corneal Keratopathy | 4 | (2) | 2 | (1) | - | - | - | - | ||||
| Glaucoma | 3 | (1.5) | 2 | (1) | 1 | (<1) | - | - | 1 | (<1) | ||
| Abnormal Vision/Diplopia | - | - | - | - | 3 | (1.5) | - | |||||
| Thromboembolic | ||||||||||||
| Pulmonary Embolism | 4 | (2) | 2 | (1) | 1 | (<1) | - | - | 1 | (<1) | ||
| Thrombophlebitis | - | 2 | (1) | 1 | (<1) | 1 | (<1) | 4 | (2) | 3 | (1.5) | |
| Thrombosis | - | 1 | (<1) | 1 | (<1) | - | 3 | (1.5) | 4 | (2) | ||
| CVA/TIA | 1 | (<1) | - | - | 1 | (<1) | 4 | (2) | 4 | (2) | ||
| Elevated Liver Tests** | ||||||||||||
| SGOT | 11 | (5) | 4 | (2) | 30 | (19) | 22 | (15) | 32 | (15) | 35 | (17) |
| Alkaline Phosphatase | 41 | (19) | 24 | (11) | 16 | (10) | 13 | (9) | 18 | (8) | 31 | (15) |
| Bilirubin | 3 | (1.5) | 4 | (2) | 2 | (1) | 1 | (<1) | 2 | (1) | 3 | (1.5) |
| Hypercalcemia | 6 | (3) | 6 | (3) | 1 | (<1) | - | - | - | |||
Other adverse events of unclear causal relationship to FARESTON included leukopenia and thrombocytopenia, skin discoloration or dermatitis, constipation, dyspnea, paresis, tremor, vertigo, pruritis, anorexia, reversible corneal opacity (corneal verticulata), asthenia, alopecia, depression, jaundice, and rigors.
In the 200 and 240 mg FARESTON dose arms, the incidence of SGOT elevation and nausea was higher. Approximately 4% of patients were withdrawn for toxicity from the high-dose FARESTON treatment arms. Reasons for withdrawal included hypercalcemia, abnormal liver function tests, and one case each of toxic hepatitis, depression, dizziness, incoordination, ataxia, blurry vision, diffuse dermatitis, and a constellation of symptoms consisting of nausea, sweating, and tremor.
OVERDOSAGE
Lethality was observed in rats following single oral doses that were ≥1000 mg/kg (about 150 times the recommended human dose on a mg/m2 basis) and was associated with gastric atony/dilatation leading to interference with digestion and adrenal enlargement.
Vertigo, headache, and dizziness were observed in healthy volunteer studies at a daily dose of 680 mg for 5 days. The symptoms occurred in two of the five subjects during the third day of the treatment and disappeared within 2 days of discontinuation of the drug. No immediate concomitant changes in any measured clinical chemistry parameters were found. In a study in postmenopausal breast cancer patients, toremifene 400 mg/m2/day caused dose-limiting nausea, vomiting, and dizziness, as well as reversible hallucinations and ataxia in one patient.
Theoretically, overdose may be manifested as an increase of antiestrogenic effects, such as hot flashes; estrogenic effects, such as vaginal bleeding; or nervous system disorders, such as vertigo, dizziness, ataxia, and nausea. There is no specific antidote and the treatment is symptomatic.
DOSAGE AND ADMINISTRATION
The dosage of FARESTON is 60 mg, once daily, orally. Treatment is generally continued until disease progression is observed.
HOW SUPPLIED
FARESTON Tablets, containing toremifene citrate in an amount equivalent to 60 mg of toremifene, are round, convex, unscored, uncoated, and white, or almost white. FARESTON Tablets are identified with TO 60 embossed on one side. FARESTON Tablets are available as:
NDC 11399-005-30 bottles of 30
NDC 11399-005-01 bottles of 100
Store at 25°C (77°F)
excursions permitted to 15-30°C (59-86°F)
[see USP Controlled Room Temperature].
Protect from heat and light.
Distributed by GTx, Inc.
Memphis, TN 38163, USA
Product covered by Orion Product Patents and related patent numbers.
© 2004 GTx, Inc.
All rights reserved.
1E Rev. 12/2004
| Fareston (toremifene citrate) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
Revised: 08/2007GTx, Inc.