EDARBI
-
azilsartan medoxomil tablet
Takeda Pharmaceuticals America, Inc.
----------
|
||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: AVOID USE IN PREGNANCY
When pregnancy is detected, discontinue Edarbi as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Edarbi may be used alone or in combination with other antihypertensive agents.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose in adults is 80 mg taken orally once daily. Consider a starting dose of 40 mg for patients who are treated with high doses of diuretics.
If blood pressure is not controlled with Edarbi alone, additional blood pressure reduction can be achieved by taking Edarbi with other antihypertensive agents.
Edarbi may be taken with or without food [see Clinical Pharmacology (12.3)].
2.2 Handling Instructions
Do not repackage Edarbi. Dispense and store Edarbi in its original container to protect Edarbi from light and moisture.
2.3 Special Populations
No initial dose adjustment is recommended for elderly patients, patients with mild-to-severe renal impairment, end-stage renal disease, or mild-to-moderate hepatic dysfunction. Edarbi has not been studied in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
Edarbi is supplied as white to nearly white round tablets in the following dosage strengths:
- 40 mg tablets - debossed "ASL" on one side and "40" on the other
- 80 mg tablets - debossed "ASL" on one side and "80" on the other
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Fetal/Neonatal Morbidity and Mortality
Drugs that act directly on the renin-angiotensin system can cause fetal and neonatal morbidity and death when administered to pregnant women during the second and third trimester. When pregnancy is detected, Edarbi should be discontinued as soon as possible.
The use of drugs that act directly on the renin-angiotensin system during the second and third trimesters of pregnancy has been associated with fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death. Oligohydramnios has also been reported, presumably resulting from decreased fetal renal function; oligohydramnios in this setting has been associated with fetal limb contractures, craniofacial deformation, and hypoplastic lung development. Prematurity, intrauterine growth retardation, and patent ductus arteriosus have also been reported, although it is not clear whether these occurrences were due to exposure to the drug.
These adverse effects do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester. Mothers whose embryos and fetuses are exposed to an angiotensin II receptor antagonist only during the first trimester should be so informed. Nonetheless, when patients become pregnant, physicians should have the patient discontinue the use of Edarbi as soon as possible.
Rarely (probably less often than once in every thousand pregnancies), no alternative to a drug acting on the renin-angiotensin system is available. In these rare cases, the mother should be apprised of the potential hazards to the fetus and serial ultrasound examinations should be performed to assess the intra-amniotic environment.
If oligohydramnios is observed, Edarbi should be discontinued unless it is considered life-saving for the mother. Contraction stress testing, a nonstress test or biophysical profiling may be appropriate, depending upon the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
Infants with histories of in utero exposure to an angiotensin II receptor antagonist should be closely observed for hypotension, oliguria, and hyperkalemia. If oliguria occurs, attention should be directed toward support of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required as a means of reversing hypotension and/or substituting for impaired renal function.
5.2 Hypotension in Volume- or Salt-Depleted Patients
In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (eg, those being treated with high doses of diuretics), symptomatic hypotension may occur after initiation of treatment with Edarbi. Correct volume or salt depletion prior to administration of Edarbi, or start treatment at 40 mg. If hypotension does occur, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.3 Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin system, changes in renal function may be anticipated in susceptible individuals treated with Edarbi. In patients whose renal function may depend on the activity of the renin-angiotensin system (e.g., patients with severe congestive heart failure, renal artery stenosis, or volume depletion), treatment with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers has been associated with oliguria or progressive azotemia and rarely with acute renal failure and death. Similar results may be anticipated in patients treated with Edarbi [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen have been reported. There has been no long-term use of Edarbi in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 4814 patients were evaluated for safety when treated with Edarbi at doses of 20, 40 or 80 mg in clinical trials. This includes 1704 patients treated for at least 6 months; of these, 588 were treated for at least 1 year.
Treatment with Edarbi was well-tolerated with an overall incidence of adverse reactions similar to placebo. The rate of withdrawals due to adverse events in placebo-controlled monotherapy and combination therapy trials was 2.4 % (19/801) for placebo, 2.2% (24/1072) for Edarbi 40 mg, and 2.7% (29/1074) for Edarbi 80 mg. The most common adverse event leading to discontinuation, hypotension/orthostatic hypotension, was reported by 0.4% (8/2146) patients randomized to Edarbi 40 mg or 80 mg compared to 0% (0/801) patients randomized to placebo. Generally, adverse reactions were mild, not dose related and similar regardless of age, gender and race.
In placebo controlled monotherapy trials, diarrhea was reported up to 2% in patients treated with Edarbi 80 mg daily compared with 0.5% of patients on placebo.
Other adverse reactions with a plausible relationship to treatment that have been reported with an incidence of ≥0.3% and greater than placebo in more than 3300 patients treated with Edarbi in controlled trials are listed below:
Gastrointestinal Disorders: nausea
General Disorders and Administration Site Conditions: asthenia, fatigue
Musculoskeletal and Connective Tissue Disorders: muscle spasm
Nervous System Disorders: dizziness, dizziness postural
Respiratory, Thoracic and Mediastinal Disorders: cough
6.2 Clinical Laboratory Findings
In controlled clinical trials, clinically relevant changes in standard laboratory parameters were uncommon with administration of Edarbi.
Serum creatinine: Small reversible increases in serum creatinine are seen in patients receiving 80 mg of Edarbi. The increase may be larger when coadministered with chlorthalidone or hydrochlorothiazide.
In addition, patients taking Edarbi who had moderate to severe renal impairment at baseline or who were >75 years of age were more likely to report serum creatinine increases.
Hemoglobin/Hematocrit: Low hemoglobin, hematocrit, and RBC counts were observed in 0.2%, 0.4%, and 0.3% of Edarbi-treated subjects, respectively. None of these abnormalities were reported in the placebo group. Low and high markedly abnormal platelet and WBC counts were observed in <0.1% of subjects.
7 DRUG INTERACTIONS
No clinically significant drug interactions have been observed in studies of azilsartan medoxomil or azilsartan given with amlodipine, antacids, chlorthalidone, digoxin, fluconazole, glyburide, ketoconazole, metformin, pioglitazone, and warfarin. Therefore, Edarbi may be used concomitantly with these medications.
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors
(COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or who have compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including azilsartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving azilsartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including azilsartan, may be attenuated by NSAIDs, including selective COX-2 inhibitors.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C (first trimester) and D (second and third trimesters). There is no clinical experience with the use of Edarbi in pregnant women [see Warnings and Precautions (5.1)].
8.3 Nursing Mothers
It is not known if azilsartan is excreted in human milk, but azilsartan is excreted at low concentrations in the milk of lactating rats. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
No dose adjustment with Edarbi is necessary in elderly patients. Of the total patients in clinical studies with Edarbi, 26% were elderly (65 years of age and older); 5% were 75 years of age and older. Abnormally high serum creatinine values were more likely to be reported for patients age 75 or older. No other differences in safety or effectiveness were observed between elderly patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dose adjustment is not required in patients with mild-to-severe renal impairment or end-stage renal disease. Patients with moderate to severe renal impairment are more likely to report abnormally high serum creatinine values.
8.7 Hepatic Impairment
No dose adjustment is necessary for subjects with mild or moderate hepatic impairment. Edarbi has not been studied in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Limited data are available related to overdosage in humans. During controlled clinical trials in healthy subjects, once daily doses up to 320 mg of Edarbi were administered for 7 days and were well tolerated. In the event of an overdose, supportive therapy should be instituted as dictated by the patient's clinical status. Azilsartan is not dialyzable [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist.
The drug substance used in the drug product formulation is the potassium salt of azilsartan medoxomil, also known by the US accepted name of azilsartan kamedoxomil and is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate monopotassium salt.
Its empirical formula is C30H23KN4O8 and its structural formula is:
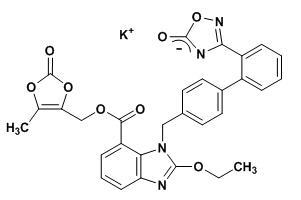
Azilsartan kamedoxomil is a white to nearly white powder with a molecular weight of 606.62. It is practically insoluble in water and freely soluble in methanol.
Edarbi is available for oral use as tablets. The tablets have a characteristic odor. Each Edarbi tablet contains 42.68 or 85.36 mg of azilsartan kamedoxomil, which is equivalent to containing 40 mg or 80 mg respectively, of azilsartan medoxomil and the following inactive ingredients: mannitol, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzymes (ACE, kinase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Azilsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathway for angiotensin II synthesis.
An AT2 receptor is also found in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Azilsartan has more than a 10,000-fold greater affinity for the AT1 receptor than for the AT2 receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction catalyzed by ACE. Because azilsartan does not inhibit ACE (kinase II), it should not affect bradykinin levels. Whether this difference has clinical relevance is not yet known. Azilsartan does not bind to or block other receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of azilsartan on blood pressure.
12.2 Pharmacodynamics
Azilsartan inhibits the pressor effects of an angiotensin II infusion in a dose-related manner. An azilsartan single dose equivalent to 32 mg azilsartan medoxomil inhibited the maximal pressor effect by approximately 90% at peak, and approximately 60% at 24 hours. Plasma angiotensin I and II concentrations and plasma renin activity increased while plasma aldosterone concentrations decreased after single and repeated administration of Edarbi to healthy subjects; no clinically significant effects on serum potassium or sodium were observed.
Effect on Cardiac Repolarization: A thorough QT/QTc study was conducted to assess the potential of azilsartan to prolong the QT/QTc interval in healthy subjects. There was no evidence of QT/QTc prolongation at a dose of 320 mg of Edarbi.
12.3 Pharmacokinetics
Absorption: Azilsartan medoxomil is hydrolyzed to azilsartan, the active metabolite, in the gastrointestinal tract during absorption. Azilsartan medoxomil is not detected in plasma after oral administration. Dose proportionality in exposure was established for azilsartan in the azilsartan medoxomil dose range of 20 mg to 320 mg after single or multiple dosing.
The estimated absolute bioavailability of azilsartan following administration of azilsartan medoxomil is approximately 60%. After oral administration of azilsartan medoxomil, peak plasma concentrations (Cmax) of azilsartan are reached within 1.5 to 3 hours. Food does not affect the bioavailability of azilsartan.
Distribution: The volume of distribution of azilsartan is approximately 16L. Azilsartan is highly bound to human plasma proteins (>99%), mainly serum albumin. Protein binding is constant at azilsartan plasma concentrations well above the range achieved with recommended doses.
In rats, minimal azilsartan-associated radioactivity crossed the blood-brain barrier. Azilsartan passed across the placental barrier in pregnant rats and was distributed to the fetus.
Metabolism and Elimination: Azilsartan is metabolized to two primary metabolites. The major metabolite in plasma is formed by O-dealkylation, referred to as metabolite M-II, and the minor metabolite is formed by decarboxylation, referred to as metabolite M-I. Systemic exposures to the major and minor metabolites in humans were approximately 50% and less than 1% of azilsartan, respectively. M-I and M-II do not contribute to the pharmacologic activity of Edarbi. The major enzyme responsible for azilsartan metabolism is CYP2C9.
Following an oral dose of 14C-labeled azilsartan medoxomil, approximately 55% of radioactivity was recovered in feces and approximately 42% in urine, with 15% of the dose excreted in urine as azilsartan. The elimination half-life of azilsartan is approximately 11 hours and renal clearance is approximately 2.3 mL/min. Steady-state levels of azilsartan are achieved within 5 days and no accumulation in plasma occurs with repeated once-daily dosing.
Special Populations
The effect of demographic and functional factors on the pharmacokinetics of azilsartan was studied in single and multiple dose studies. Pharmacokinetic measures indicating the magnitude of the effect on azilsartan are presented in Figure 1 as change relative to reference (test/reference). Effects are modest and do not call for dosage adjustment.
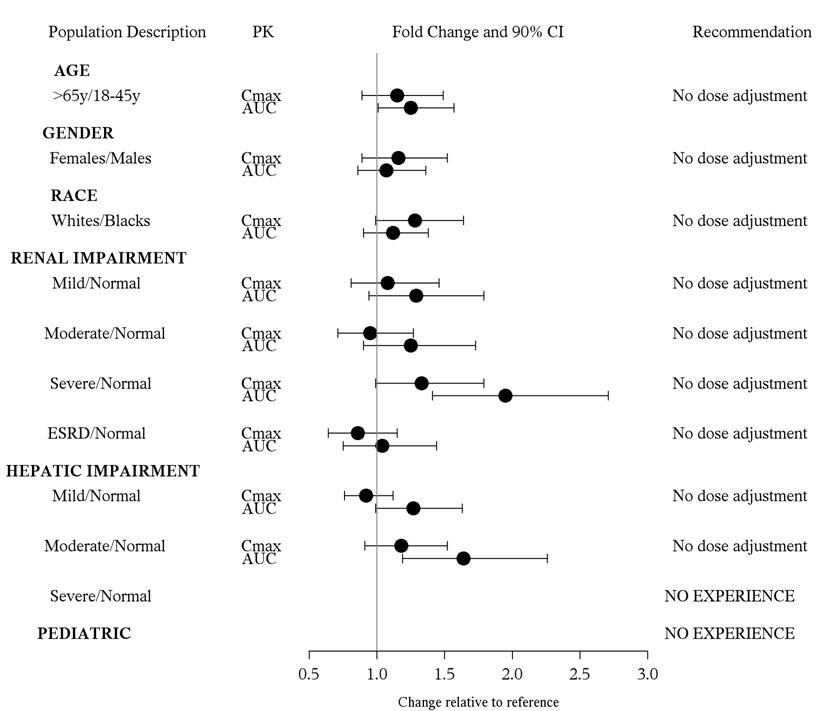 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Azilsartan medoxomil was not carcinogenic when assessed in 26-week transgenic (Tg.rasH2) mouse and 2-year rat studies. The highest doses tested (450 mg azilsartan medoxomil/kg/day in the mouse and 600 mg azilsartan medoxomil/kg/day in the rat) produced exposures to azilsartan that are 12 (mice) and 27 (rats) times the average exposure to azilsartan in humans given the maximum recommended human dose (MRHD, 80 mg azilsartan medoxomil/day). M-II was not carcinogenic when assessed in 26-week Tg.rasH2 mouse and 2-year rat studies. The highest doses tested (approximately 8000 mg M-II/kg/day (males) and 11,000 mg M-II/kg/day (females) in the mouse and 1000 mg M-II/kg/day (males) and up to 3000 mg M-II/kg/day (females) in the rat) produced exposures that are, on average, about 30 (mice) and 7 (rats) times the average exposure to M-II in humans at the MRHD.
Mutagenesis: Azilsartan medoxomil, azilsartan, and M-II were positive for structural aberrations in the Chinese Hamster Lung Cytogenetic Assay. In this assay, structural chromosomal aberrations were observed with the prodrug, azilsartan medoxomil, without metabolic activation. The active moiety, azilsartan was also positive in this assay both with and without metabolic activation. The major human metabolite, M-II was also positive in this assay during a 24 hr assay without metabolic activation.
Azilsartan medoxomil, azilsartan, and M-II were devoid of genotoxic potential in the Ames reverse mutation assay with Salmonella typhimurium and Escherichia coli, the in vitro Chinese Hamster Ovary Cell forward mutation assay, the in vitro mouse lymphoma (tk) gene mutation test, the ex vivo unscheduled DNA synthesis test, and the in vivo mouse and/or rat bone marrow micronucleus assay.
Impairment of Fertility: There was no effect of azilsartan medoxomil on the fertility of male or female rats at oral doses of up to 1000 mg azilsartan medoxomil/kg/day [6000 mg/m2 (approximately 122 times the MRHD of 80 mg azilsartan medoxomil/60 kg on a mg/m2 basis)]. Fertility of rats also was unaffected at doses of up to 3000 mg M-II/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology: In peri- and postnatal rat development studies, adverse effects on pup viability, delayed incisor eruption and dilatation of the renal pelvis along with hydronephrosis were seen when azilsartan medoxomil was administered to pregnant and nursing rats at 1.2 times the MRHD on a mg/m2 basis. Reproductive toxicity studies indicated that azilsartan medoxomil was not teratogenic when administered at oral doses up to 1000 mg azilsartan medoxomil/kg/day to pregnant rats (122 times the MRHD on a mg/m2 basis) or up to 50 mg azilsartan medoxomil/kg/day to pregnant rabbits (12 times the MRHD on a mg/m2 basis). M-II also was not teratogenic in rats or rabbits at doses up to 3000 mg M-II/kg/day. Azilsartan crossed the placenta and was found in the fetuses of pregnant rats and was excreted into the milk of lactating rats.
14 CLINICAL STUDIES
The antihypertensive effects of Edarbi have been demonstrated in a total of 7 double-blind, randomized studies, which included 5 placebo-controlled and 4 active comparator-controlled studies (not mutually exclusive). The studies ranged from 6 weeks to 6 months in duration, at doses ranging from 20 mg to 80 mg once daily. A total of 5941 patients (3672 given Edarbi, 801 given placebo, and 1468 given active comparator) with mild, moderate or severe hypertension were studied. Overall, 51% of patients were male and 26% were 65 years or older; 67% were white and 19% were black.
Two 6-week randomized, double blind studies compared the effect on blood pressure of Edarbi at doses of 40 mg and 80 mg, with placebo and with active comparators. Blood pressure reductions compared to placebo based on clinic blood pressure measurements at trough and 24 hour mean blood pressure by ambulatory blood pressure monitoring (ABPM) are shown in Table 1 for both studies. Edarbi, 80 mg, was statistically superior to placebo and active comparators for both clinic and 24 hour mean blood pressure measurements.
| Study 1 N=1285 | Study 2 N=989 |
|||
|---|---|---|---|---|
| Clinic Blood Pressure (Mean Baseline 157.4 / 92.5) | 24 Hour Mean by ABPM (Mean Baseline 144.9 / 88.7) | Clinic Blood Pressure (Mean Baseline 159.0 / 91.8) | 24 Hour Mean by ABPM (Mean Baseline 146.2 / 87.6) |
|
| Edarbi 40 mg | -14.6 / -6.2 | -13.2 / -8.6 | -12.4 / -7.1 | -12.1 / -7.7 |
| Edarbi 80 mg | -14.9 / -7.5 | -14.3 / -9.4 | -15.5 / -8.6 | -13.2 / -7.9 |
| Olmesartan 40 mg | -11.4 / -5.3 | -11.7 / -7.7 | -12.8 / -7.1 | -11.2 / -7.0 |
| Valsartan 320 mg | -9.5 / -4.4 | -10.0 / -7.0 | ||
In a study comparing Edarbi to valsartan over 24 weeks, similar results were observed.
Most of the antihypertensive effect occurs within the first 2 weeks of dosing.
Figure 2 shows the 24-hour ambulatory systolic and diastolic blood pressure profiles at endpoint.
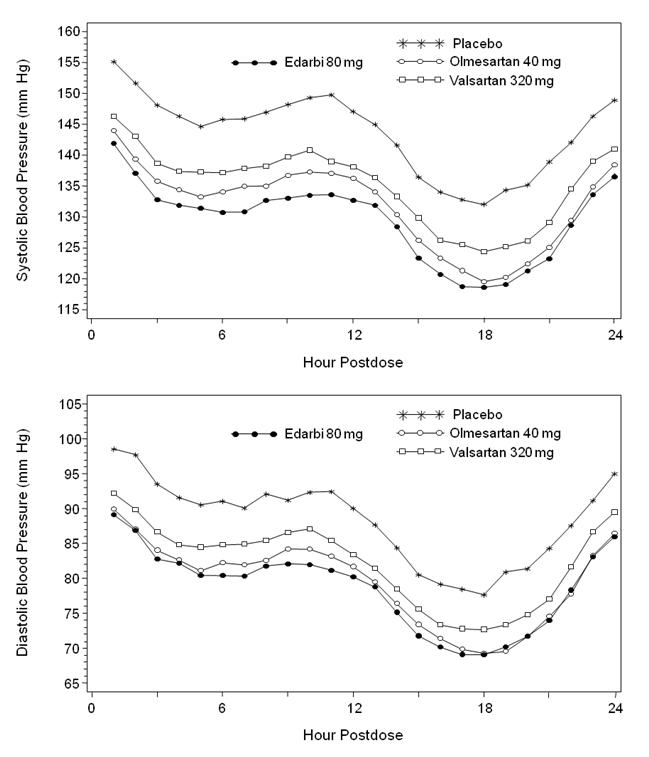 |
Other studies showed similar 24 hour ambulatory blood pressure profiles.
Edarbi has a sustained and consistent antihypertensive effect during long-term treatment, as shown in a study that randomized patients to placebo or continued Edarbi after 26 weeks. No rebound effect was observed following the abrupt cessation of Edarbi therapy.
Edarbi was effective in reducing blood pressure regardless of the age, gender, or race of patients, but the effect, as monotherapy, was smaller, approximately half, in black patients, who tend to have low renin levels. This has been generally true for other angiotensin II antagonists and ACE inhibitors.
Edarbi has about its usual blood pressure lowering effect size when added to a calcium channel blocker (amlodipine) or a thiazide-type diuretic (chlorthalidone).
There are no trials of Edarbi demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
16 HOW SUPPLIED/STORAGE AND HANDLING
Edarbi tablets are unscored and white to nearly white, debossed with "ASL" on one side and "40" or "80" on the other.
| Tablet | NDC 64764-xxx-xx | |
|---|---|---|
| Bottle/30 | Bottle/90 | |
| 40 mg | 844-30 | 844-90 |
| 80 mg | 884-30 | 884-90 |
Store at 25°C (77°F), excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from moisture and light. Do not repackage; dispense and store in original container.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (17.2).
17.1 General Information
Pregnancy: Female patients of childbearing age should be told that use of drugs like Edarbi that act on the renin-angiotensin system during pregnancy can cause serious problems in the fetus and infant including low blood pressure, poor development of skull bones, kidney failure, and death. These consequences do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester. Discuss other treatment options with female patients planning to become pregnant. Women using Edarbi who become pregnant should notify their physicians as soon as possible [see FDA-Approved Patient Labeling (17.2)].
17.2 FDA-Approved Patient Labeling
Patient Information
Edarbi (eh-DAR-bee)
(azilsartan medoxomil)
Tablets
Read this Patient Information leaflet before you start taking Edarbi and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about Edarbi?
Taking Edarbi during your second and third trimester can cause harm and even death to your unborn baby. If you think that you are pregnant, stop taking Edarbi and tell your doctor right away. If you plan to become pregnant, talk to your doctor about other ways to lower your blood pressure.
What is Edarbi?
Edarbi is a prescription medicine called an angiotensin II receptor blocker (ARB) used to treat high blood pressure (hypertension) in adults.
Your doctor may prescribe other medicines for you to take along with Edarbi to treat your high blood pressure.
It is not known if Edarbi is safe and effective in children under 18 years of age.
What should I tell my doctor before taking Edarbi?
Before you take Edarbi, tell your doctor if you:
- have been told that you have abnormal body salt (electrolytes) levels in your blood
- are pregnant or plan to become pregnant. See "What is the most important information I should know about Edarbi?"
- are breastfeeding or plan to breastfeed. It is not known if Edarbi passes into your breast milk. You and your doctor should decide if you will take Edarbi or breastfeed. You should not do both. Talk with your doctor about the best way to feed your baby if you take Edarbi.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Especially tell your doctor if you take:
- other medicines used to treat your high blood pressure or heart problem
- water pills (diuretic)
Ask your doctor if you are not sure if you are taking a medicine listed above.
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I take Edarbi?
- Your doctor will tell you how much Edarbi to take and when to take it. Follow his/her instructions.
- Edarbi can be taken with or without food.
- If you take too much Edarbi, call your doctor, or go to the nearest hospital emergency room right away.
What are the possible side effects of Edarbi?
Edarbi may cause side effects, including:
- Harm or death to your unborn fetus if taken in the second or third trimester. See "What is the most important information I should know about Edarbi?"
-
Low blood pressure (hypotension) and dizziness is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- take other medicines that affect your blood pressure
- get sick with vomiting or diarrhea
- do not drink enough fluids
- If you feel faint or dizzy, lie down and call your doctor right away.
These are not all the possible side effects with Edarbi. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store Edarbi?
- Store Edarbi at 59°F to 86°F (15°C to 30°C).
- Store Edarbi in the original container that you received from your pharmacist or doctor. Do not put Edarbi into a different container.
- Keep Edarbi in a tightly closed container, and keep Edarbi out of the light.
Keep Edarbi and all medicines out of the reach of children.
General information about Edarbi.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not give Edarbi to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Edarbi. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Edarbi that is written for health professionals.
For more information, go to www.edarbi.com or call 1-877-825-3327.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too great.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. Edarbi tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure may lower your chance of having a stroke or heart attack.
What are the ingredients in Edarbi?
Active ingredient: azilsartan medoxomil
Inactive ingredients: mannitol, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate.
Distributed by
Takeda Pharmaceuticals America, Inc.
Deerfield, IL 60015
Edarbi is a trademark of Takeda Pharmaceutical Company Limited registered with the U.S. Patent and Trademark Office and used under license by Takeda Pharmaceuticals America, Inc.
©2011 Takeda Pharmaceuticals America, Inc.
April 2011
AZL069 R2
PRINCIPAL DISPLAY PANEL - 40 mg Tablet Carton
Professional Sample-
Not for Sale
NDC 64764-844-07
Contains one
patient sample unit
of 7 tablets each.
edarbi
(azilsartan medoxomil)
tablets
40 mg per tablet
Each white tablet contains:
42.68 mg azilsartan kamedoxomil (equivalent to
40 mg azilsartan medoxomil).
Usual Dosage: See package insert.
Store at 25°C (77°F); excursions permitted to
15-30°C (59-86°F) [see USP Controlled Room
Temperature].
Protect from moisture and light.
Dispense and store in original container.
Rx Only
Takeda
Distributed by:
Takeda Pharmaceuticals
America, Inc.
Deerfield, IL 60015
C081-01
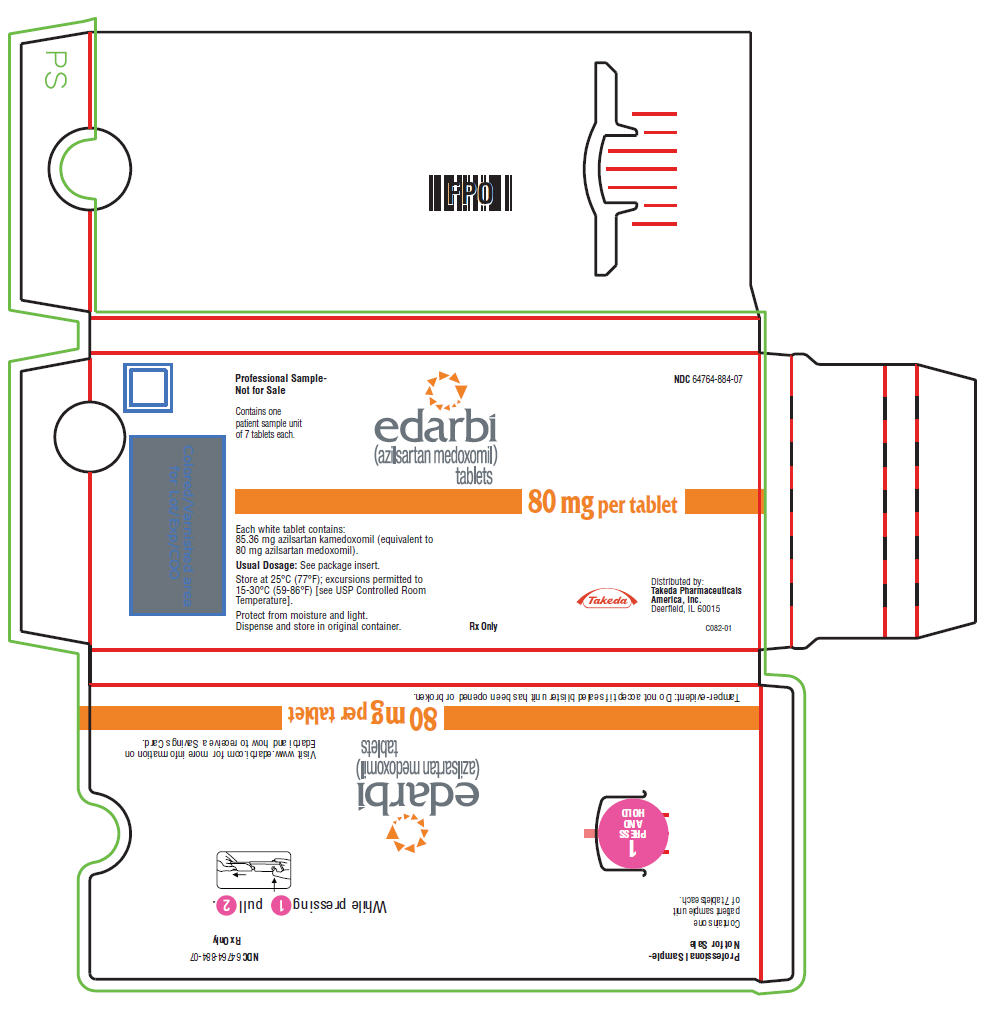
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Carton
Professional Sample-
Not for Sale
NDC 64764-884-07
Contains one
patient sample unit
of 7 tablets each.
edarbi
(azilsartan medoxomil)
tablets
80 mg per tablet
Each white tablet contains:
85.36 mg azilsartan kamedoxomil (equivalent to
80 mg azilsartan medoxomil).
Usual Dosage: See package insert.
Store at 25°C (77°F); excursions permitted to
15-30°C (59-86°F) [see USP Controlled Room
Temperature].
Protect from moisture and light.
Dispense and store in original container.
Rx Only
Takeda
Distributed by:
Takeda Pharmaceuticals
America, Inc.
Deerfield, IL 60015
C082-01
| EDARBI
azilsartan medoxomil tablet |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA200796 | 02/25/2011 | |
| EDARBI
azilsartan medoxomil tablet |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA200796 | 02/25/2011 | |
| Labeler - Takeda Pharmaceuticals America, Inc. (830134016) |