KERATOL 40
-
urea cream
Breckenridge Pharmaceutical, Inc.
----------
Keratol™ 40Cream, Lotion or Gel
(40% Urea)
Rx Only
For external use only. Not for ophthalmic use.
DESCRIPTION
Keratol™ 40 is a keratolytic, emollient which is a gentle, yet potent, tissue softener for nails and/or skin.
Each gram of Keratol™ 40 Cream and Keratol™ 40 Lotion contains: 40% Urea. Other ingredients: Carbomer 940, Cetearyl Alcohol (and) Ceteareth 20, Cetyl Alcohol, Mineral Oil, Polyethylene Glycol 300, Petrolatum, Purified Water, and Triethanolamine. Each gram of Keratol™ 40 Gel contains: 40% Urea. Other ingredients: Edetate Disodium, Glycerin, Hydroxyethyl Cellulose, PEG-6 Caprylic/Capric Glycerides, and Purified Water. Urea is a diamide of carbonic acid with the following chemical structure:
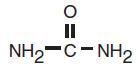
CLINICAL PHARMACOLOGY
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual debridement of the nail plate.
PHARMACOKINETICS
The mechanism of action of topically applied Urea is not yet known.
INDICATIONS AND USES
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or prurient debris, or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, ingrown, and devitalized nails.
CONTRAINDICATIONS
Known hypersensitivity to any of the listed ingredients.
WARNINGS
For external use only. Avoid contact with eyes, lips or mucous membranes.
PRECAUTIONS
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY
Pregnancy Category B
Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Keratol™ 40 should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Keratol™ 40 is administered to a nursing woman.
ADVERSE REACTIONS
Transient stinging, burning, itching, or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION
Apply Keratol™ 40 to affected skin twice per day, or as directed by a physician. Rub in until completely absorbed. Apply to diseased or damaged nail tissue twice per day, or as directed by a physician.
HOW SUPPLIED
Keratol™ 40 Cream (40%)
28.4 g (1 oz.) tube, NDC 51991-268-41
85.2 g (3 oz.) tube, NDC 51991-268-19
198.6 g (7 oz.) tube, NDC 51991-268-37
Keratol™ 40 Lotion (40%)
8 fl.oz. (237mL) Bottle NDC 51991-270-18
Keratol™ 40 Gel (40%)
15 mL(1/2 oz.) Bottle NDC 51991-269-15
Dispense in original container.
Store up to 25°C (77°F); excursions permitted to 15°-30° C (59°-86°F). See USP Controlled Room Temperature.
Protect from freezing.
Warning: Keep this and all medications out of the reach of children. In case of accidental overdose seek professional assistance or contact a poison control center immediately.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Distributed by: Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
Manufactured by: DSC Laboratories
Muskegon, MI 49442
Rev. 1/08
How to properly use
Keratol™ 40
(40% Urea) Rx Only
Gentle, yet potent, tissue-softener for skin or nails.
Easy steps to gently thin and soften thick, rough or dry skin seen, to various degrees, in psoriasis, xerosis, ichthyosis, keratosis, keratoderma, dermatitis, pruritus, eczema and calluses as well as damaged, diseased, or devitalized and ingrown nails.
For skin tissue:
1. Apply Keratol™ 40 to affected skin tissue twice per day, or as directed by a physician.
2. Rub in until completely absorbed.
For nail tissue:
1. Apply Keratol™ 40 to affected nail tissue twice per day, or as directed by a physician.
2. Let dry uncovered or apply and cover with adhesive bandage or gauze secured with adhesive tape.
Available as:
Keratol™ 40 (40% Urea) Cream, Lotion or Gel
PRINCIPAL DISPLAY PANEL - 85 g Carton
Breckenridge
Pharmaceutical, Inc.
NDC 51991-268-19
Keratol™ 40 Cream
(40% Urea)
Net Wt. 3 oz. (85.0 g)
Rx Only
For Topical Use Only

| KERATOL 40
urea cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 03/01/2005 | 04/30/2011 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DSC Labs | 097807374 | MANUFACTURE | |