FOLLISTIM AQ
-
follitropin injection, solution
Organon Pharmaceuticals USA
----------
|
|||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Follistim® AQ (follitropin beta injection) Cartridge is indicated:
In Women for:
1.1 Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure.
Prior to initiation of treatment with Follistim AQ Cartridge:
- Women should have a complete gynecologic and endocrinologic evaluation
- Primary ovarian failure should be excluded
- The possibility of pregnancy should be excluded
- Tubal patency should be demonstrated
- The fertility status of the male partner should be evaluated
1.2 Development of multiple follicles in ovulatory women participating in an Assisted Reproductive Technology (ART) program.
Prior to initiation of treatment with Follistim AQ Cartridge:
- Women should have a complete gynecologic and endocrinologic evaluation and diagnosis of cause of infertility
- The possibility of pregnancy should be excluded
- The fertility status of the male partner should be evaluated
In Men for:
1.3 Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure
Prior to initiation of treatment with Follistim AQ Cartridge:
- Men should have a complete medical and endocrinologic evaluation
- Hypogonadotropic hypogonadism should be confirmed and primary testicular failure should be excluded
- Serum testosterone levels should be normalized with human chorionic gonadotropin (hCG) treatment.
- The fertility status of the female partner should be evaluated
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If the solution is not clear and colorless or has particles in it, the solution should not be used
- Do not add any other medicines into the Follistim AQ Cartridge
- Follistim AQ Cartridge with the pen injector device delivers on average an 18% higher amount of follitropin beta when compared to reconstituted Follistim delivered with a conventional syringe and needle. When administering Follistim AQ Cartridge, a lower starting dose and lower dose adjustments (as compared to reconstituted Follistim) should be considered. For that purpose the following Dose Conversion Table is provided:
| Lyophilized recombinant FSH dosing in ampules or vials, using conventional syringe | Follistim AQ Cartridge dosing with the Follistim Pen |
|---|---|
|
|
| 75 IU | 50 IU |
| 150 IU | 125 IU |
| 225 IU | 175 IU |
| 300 IU | 250 IU |
| 375 IU | 300 IU |
| 450 IU | 375 IU |
2.2 Recommended Dosing for Ovulation Induction
The dosing scheme is stepwise and is individualized for each woman [see Clinical Studies (14.1)].
- A starting daily dose of 50 international units of Follistim AQ Cartridge is administered [see Dosage and Administration (2.1)] subcutaneously daily for at least the first 7 days
- Subsequent dosage adjustments are made at weekly intervals based upon ovarian response. If an increase in dose is indicated by the ovarian response, the increase should be made by 25 or 50 international units of Follistim AQ Cartridge at weekly intervals until follicular growth and/or serum estradiol levels indicate an adequate ovarian response
The following should be considered when planning the woman's individualized dose:- Appropriate Follistim AQ Cartridge dose adjustment(s) should be used to prevent multiple follicular growth and cycle cancellation
- The maximum, individualized, daily dose of Follistim AQ Cartridge is 250 international units
- Treatment should continue until ultrasonic visualizations and/or serum estradiol determinations approximate the pre-ovulatory conditions seen in normal individuals
- When pre-ovulatory conditions are reached, 5000 to 10,000 international units of hCG are used to induce final oocyte maturation and ovulation.
The administration of hCG must be withheld in cases where the ovarian monitoring suggests an increased risk of OHSS on the last day of Follistim AQ Cartridge therapy [see Warnings and Precautions (5.1, 5.2, 5.10)] - The woman and her partner should be encouraged to have intercourse daily, beginning on the day prior to the administration of hCG and until ovulation becomes apparent [see Warnings and Precautions (5.10)]
- During treatment with Follistim AQ Cartridge and during a two-week post-treatment period, the woman should be assessed at least every other day for signs of excessive ovarian stimulation.
It is recommended that Follistim AQ Cartridge administration be stopped if the ovarian monitoring suggests an increased risk of OHSS or abdominal pain occurs. Most OHSS occurs after treatment has been discontinued and reaches its maximum at about seven to ten days post-ovulation
2.3 Recommended Dosing for ART
The dosing scheme follows a stepwise approach and is individualized for each woman.
- A starting dose of 125 to 175 international units of Follistim AQ Cartridge is administered [see Dosage and Administration (2.1)] subcutaneously daily for at least the first 4 days of treatment
- Subsequent dosing beyond the first 4 days of treatment is adjusted based upon the woman's ovarian response as determined by ultrasound evaluation of follicular growth and serum estradiol levels
The following should be considered when planning the woman's individualized dose:- For most normal responding women, the daily starting dose can be continued until pre-ovulatory conditions are achieved (six to twelve days)
- For low or poor responding women, the daily dose should be increased according to the ovarian response. The maximum, individualized, daily dose of Follistim AQ Cartridge is 500 international units
- For high responding women [those at particular risk of abnormal ovarian enlargement and/or ovarian hyperstimulation syndrome (OHSS)], decrease or temporarily stop the daily dose, or discontinue the cycle according to individual response [see Warnings and Precautions (5.1, 5.2, 5.10)]
- When a sufficient number of follicles of adequate size are present, dosing of Follistim AQ Cartridge is stopped and final maturation of the oocytes is induced by administering hCG at a dose of 5000 to 10,000 international units. The administration of hCG should be withheld in cases where the ovarian monitoring suggests an increased risk of OHSS on the last day of Follistim AQ Cartridge therapy [see Warnings and Precautions (5.1, 5.2, 5.10)]
- Oocyte (egg) retrieval should be performed 34 to 36 hours following the administration of hCG
2.4 Recommended Dosing for Induction of Spermatogenesis in Men
- Pretreatment with hCG is required prior to concomitant therapy with Follistim AQ Cartridge and hCG. An initial dosage of 1500 international units of hCG should be administered at twice weekly intervals to normalize serum testosterone levels. If serum testosterone levels have not normalized after 8 weeks of hCG treatment, the hCG dose can be increased to 3000 international units twice weekly [see Clinical Studies (14.3)]
-
After normal serum testosterone levels have been reached, Follistim AQ Cartridge should be administered by subcutaneous injection concomitantly with hCG treatment. Follistim is given at a dosage of 450 international units per week, as either 225 international units twice weekly or 150 international units three times per week, in combination with the same hCG dose used to normalize testosterone levels. Based on delivery of a higher dose of follitropin beta with the Follistim AQ Cartridge and pen injector [see Dosage and Administration (2.1)], a lower dose of Follistim AQ Cartridge may be considered.
The concomitant therapy should be continued for at least 3 to 4 months before any improvement in spermatogenesis can be expected. If a man has not responded after this period, the combination therapy may be continued. Treatment response has been noted at up to 12 months.
3 DOSAGE FORMS AND STRENGTHS
Follistim AQ Cartridge 175 international units per 0.210 mL
Follistim AQ Cartridge 350 international units per 0.420 mL
Follistim AQ Cartridge 650 international units per 0.780 mL
Follistim AQ Cartridge 975 international units per 1.170 mL
4 CONTRAINDICATIONS
Follistim AQ Cartridge is contraindicated in women and men who exhibit:
- Prior hypersensitivity to recombinant hFSH products
- High levels of FSH indicating primary gonadal failure
- Presence of uncontrolled non-gonadal endocrinopathies (e.g., thyroid, adrenal, or pituitary disorders) [see Indications and Usage (1.1, 1.2, 1.3)]
- Hypersensitivity reactions to streptomycin or neomycin. Follistim AQ may contain traces of these antibiotics
- Tumor of the ovary, breast, uterus, testis, hypothalamus or pituitary gland
Follistim AQ Cartridge is also contraindicated in women who exhibit:
- Pregnancy [see Use in Specific Populations (8.1)]
- Heavy or irregular vaginal bleeding of undetermined origin
- Ovarian cysts or enlargement not due to polycystic ovary syndrome (PCOS)
5 WARNINGS AND PRECAUTIONS
Follistim AQ Cartridge should be used only by physicians who are experienced in infertility treatment. Follistim AQ Cartridge contains a potent gonadotropic substance capable of causing Ovarian Hyperstimulation Syndrome (OHSS) [see Warnings and Precautions (5.2)] with or without pulmonary or vascular complications [see Warnings and Precautions (5.3)] and multiple births [see Warnings and Precautions (5.5)]. Gonadotropin therapy requires the availability of appropriate monitoring facilities [see Warnings and Precautions (5.10)].
Careful attention should be given to the diagnosis of infertility and in the selection of candidates for Follistim AQ Cartridge therapy [see Indications and Usage (1.1, 1.2, 1.3) and Dosage and Administration (2.2, 2.3, 2.4)].
Switching to Follistim AQ Cartridge from other brands (manufacturer), types (recombinant, urinary, etc.), and/or methods of administration (Follistim Pen, conventional syringe, etc.) may necessitate an adjustment of the dose [see Dosage and Administration (2)].
5.1 Abnormal Ovarian Enlargement
In order to minimize the hazards associated with abnormal ovarian enlargement that may occur with Follistim AQ therapy, treatment should be individualized and the lowest effective dose should be used [see Dosage and Administration (2.2, 2.3)]. Use of ultrasound monitoring of ovarian response and/or measurement of serum estradiol levels is important to minimize the risk of overstimulation [see Warnings and Precautions (5.8)].
If the ovaries are abnormally enlarged on the last day of Follistim AQ therapy, hCG should not be administered in order to reduce the chances of developing Ovarian Hyperstimulation Syndrome (OHSS). Intercourse should be prohibited in patients with significant ovarian enlargement after ovulation because of the danger of hemoperitoneum resulting from ruptured ovarian cysts [see Warnings and Precautions (5.3)].
5.2 Ovarian Hyperstimulation Syndrome (OHSS)
OHSS is a medical entity distinct from uncomplicated ovarian enlargement and may progress rapidly to become a serious medical condition. OHSS is characterized by a dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of OHSS developing are severe pelvic pain, nausea, vomiting, and weight gain. Abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria have been reported with OHSS. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic reactions [see Warnings and Precautions (5.3)]. Transient liver function test abnormalities suggestive of hepatic dysfunction with or without morphologic changes on liver biopsy have also been reported in association with OHSS.
OHSS occurs after gonadotropin treatment has been discontinued, and it can develop rapidly, reaching its maximum about seven to ten days following treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration [see Warnings and Precautions (5.1)], the hCG must be withheld. Cases of OHSS are more common, more severe, and more protracted if pregnancy occurs; therefore, women should be assessed for the development of OHSS for at least two weeks after hCG administration.
If serious OHSS occurs, treatment should be stopped and the patient should be hospitalized. Treatment is primarily symptomatic and overall should consist of bed rest, fluid and electrolyte management, and analgesics (if needed). Because the use of diuretics can accentuate the diminished intravascular volume, diuretics should be avoided except in the late phase of resolution as described below. The management of OHSS may be divided into three phases as follows:
-
Acute Phase:
Management should be directed at preventing hemoconcentration due to loss of intravascular volume to the third space and minimizing the risk of thromboembolic phenomena and kidney damage. Fluid intake and output, weight, hematocrit, serum and urinary electrolytes, urine specific gravity, BUN and creatinine, total proteins with albumin: globulin ratio, coagulation studies, electrocardiogram to monitor for hyperkalemia, and abdominal girth should be thoroughly assessed daily or more often based on the clinical need. Treatment, consisting of limited intravenous fluids, electrolytes, and human serum albumin, is intended to normalize electrolytes while maintaining an acceptable but somewhat reduced intravascular volume. Full correction of the intravascular volume deficit may lead to an unacceptable increase in the amount of third space fluid accumulation. -
Chronic Phase:
After the acute phase is successfully managed as above, excessive fluid accumulation in the third space should be limited by instituting severe potassium, sodium, and fluid restriction. -
Resolution Phase:
As third space fluid returns to the intravascular compartment, a fall in hematocrit and increasing urinary output are observed in the absence of any increase in intake. Peripheral and/or pulmonary edema may result if the kidneys are unable to excrete third space fluid as rapidly as it is mobilized. Diuretics may be indicated during the resolution phase, if necessary, to combat pulmonary edema.
OHSS increases the risk of injury to the ovary. The ascitic, pleural, and pericardial fluid should not be removed unless there is the necessity to relieve symptoms such as pulmonary distress or cardiac tamponade. Pelvic examination may cause rupture of an ovarian cyst, which may result in hemoperitoneum, and should therefore be avoided. If bleeding occurs and requires surgical intervention, the clinical objective should be to control the bleeding and retain as much ovarian tissue as possible.
During clinical trials with Follistim therapy, OHSS occurred in 7.6% of 105 women (OI) and 5.2% of 591 women (ART) treated with Follistim.
5.3 Pulmonary and Vascular Complications
Serious pulmonary conditions (e.g., atelectasis, acute respiratory distress syndrome) have been reported in women treated with gonadotropins. In addition, thromboembolic reactions both in association with, and separate from OHSS have been reported following gonadotropin therapy. Intravascular thrombosis, which may originate in venous or arterial vessels, can result in reduced blood flow to vital organs or the extremities. Women with generally recognized risk factors for thrombosis, such as a personal or family history, severe obesity, or thrombophilia, may have an increased risk of venous or arterial thromboembolic events, during or following treatment with gonadotropins. Sequelae of such reactions have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb and rarely in myocardial infarction. In rare cases, pulmonary complications and/or thromboembolic reactions have resulted in death. In women with recognized risk factors, the benefits of ovulation induction or in vitro fertilization (IVF) treatment need to be weighed against the risks. It should be noted, that pregnancy itself also carries an increased risk of thrombosis.
5.4 Ovarian Torsion
5.5 Multi-fetal Gestation and Birth
Multi-fetal gestation and births have been reported with all gonadotropin treatments including Follistim AQ treatment. The woman and her partner should be advised of the potential risk of multi-fetal gestation and births before starting treatment.
5.6 Congenital Anomalies
5.7 Ectopic Pregnancy
Since infertile women undergoing ART, and particularly IVF, often have tubal abnormalities the incidence of ectopic pregnancies might be increased. Early confirmation of an intrauterine pregnancy should be determined by hCG testing and transvaginal ultrasound.
5.8 Spontaneous Abortion
5.9 Ovarian Neoplasms
There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have undergone multiple drug regimens for ovulation induction; however, a causal relationship has not been established.
5.10 Laboratory Tests
For Women:
In most instances, treatment with Follistim AQ will result only in follicular growth and maturation. In order to complete the final phase of follicular maturation and to induce ovulation, hCG must be given following the administration of Follistim AQ Cartridge or when clinical assessment indicates that sufficient follicular maturation has occurred. The degree of follicular maturation and the timing of hCG administration can both be determined with the use of sonographic visualization of the ovaries and endometrial lining in conjunction with measurement of serum estradiol levels. The combination of transvaginal ultrasonography and measurement of serum estradiol levels is also useful for minimizing the risk of OHSS and multi-fetal gestations.
The clinical confirmation of ovulation is obtained by the following direct or indirect indices of progesterone production as well as sonographic evidence of ovulation.
Direct or indirect indices of progesterone production are:
- Urinary or serum luteinizing hormone (LH) rise
- A rise in basal body temperature
- Increase in serum progesterone
- Menstruation following the shift in basal body temperature
The following provide sonographic evidence of ovulation:
- Collapsed follicle
- Fluid in the cul-de-sac
- Features consistent with corpus luteum formation
Sonographic evaluation of the early pregnancy is also important to rule out ectopic pregnancy.
For Men:
Clinical monitoring for spermatogenesis utilizes the following indirect or direct measures:
- Serum testosterone level
- Semen analysis
5.11 Follistim Pen
The Follistim Pen is intended only for use with Follistim AQ Cartridge. The Follistim Pen is not recommended for the blind or visually impaired without the assistance of an individual with good vision who is trained in the proper use of the injection device.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Ovarian Hyperstimulation Syndrome [see Warnings and Precautions( 5.2)]
- Atelectasis [see Warnings and Precautions (5.3)]
- Thromboembolism [see Warnings and Precautions (5.3)]
- Ovarian Torsion [see Warnings and Precautions (5.4)]
- Multi-fetal Gestation [see Warnings and Precautions (5.5)]
- Congenital Anomalies [see Warnings and Precautions (5.6)]
- Ectopic Pregnancy [see Warnings and Precautions (5.7)]
- Spontaneous Abortion [see Warnings and Precautions (5.8)]
6.1 Clinical Study Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
Ovulation Induction
In a single cycle, multi-center, assessor-blind, parallel group, comparative study, a total of 172 chronic anovulatory women who had failed to ovulate and/or conceive with clomiphene citrate therapy, were randomized and treated with Follistim (105) or a urofollitropin comparator. Adverse reactions with an incidence of greater than 2% in either treatment group are listed in Table 2.
| System Organ Class/Adverse Reactions | Treatment Number (%) of Women |
|
|---|---|---|
| Follistim N=105 n (%) | Comparator N=67 n (%) |
|
| Gastrointestinal disorders | ||
| Abdominal discomfort | 3 (2.9) | 1 (1.5) |
| Abdominal pain | 3 (2.9) | 2 (3.0) |
| Abdominal pain lower | 3 (2.9) | 1 (1.5) |
| Reproductive system and breast disorders | ||
| Ovarian cyst | 3 (2.9) | 2 (3.0) |
| Ovarian hyperstimulation syndrome | 8 (7.6) | 3 (4.5) |
| General disorders and administration site conditions | ||
| Pyrexia | 0 (0.0) | 2 (3.0) |
Adverse reactions reported commonly (greater than or equal to 2% of women treated with Follistim) in other ovulation induction clinical trials were headache, abdominal distension, constipation, diarrhea, nausea, pelvic pain, uterine enlargement, vaginal hemorrhage and injection site reaction.
The following medical events have been reported subsequent to pregnancies resulting from Follistim AQ Cartridge therapy:
- Ectopic pregnancy [see Warnings and Precautions (5.7)]
- Spontaneous abortion [see Warnings and Precautions (5.8)]
ART
In a multiple cycle, multi-center, assessor-blind, parallel group, comparative study, after pituitary suppression with a gonadotropin release hormone (GnRH) agonist, a total of 989 women were randomized and treated with Follistim (N=591) or a urofollitropin comparator as part of in vitro fertilization therapy (IVF). Adverse reactions with an incidence of greater than 2% in either treatment group are listed in Table 3.
| System Organ Class/Adverse Reactions | Treatment Number (%) of Women |
|
|---|---|---|
| Follistim N=591 n (%) | Comparator N=398 n (%) |
|
| Gastrointestinal disorders | ||
| Abdominal pain | 13 (2.2) | 4 (1.0) |
| Reproductive system and breast disorders | ||
| Ovarian hyperstimulation syndrome | 31 (5.2) | 17 (4.3) |
Adverse reactions reported commonly (greater than or equal to 2% of women treated with Follistim) in other IVF clinical trials were headache, abdominal distension, constipation, diarrhea, nausea, pelvic pain, breast tenderness, metrorrhagia, ovarian enlargement, vaginal hemorrhage, injection site reaction and rash.
The following medical events have been reported subsequent to pregnancies resulting from Follistim AQ Cartridge therapy:
- Ectopic pregnancy [see Warnings and Precautions (5.7)]
- Spontaneous abortion [see Warnings and Precautions (5.8)]
Induction of Spermatogenesis
In an open-label, non-comparative clinical trial, 49 men with hypogonadotropic hypogonadism were enrolled to received pretreatment with hCG, followed by combination therapy with hCG and Follistim for induction of spermatogenesis. Of the 49 men, 30 received weekly Follistim doses of 450 international units; 24 of these 30 men received a total of 48 weeks of treatment with Follistim. Adverse reactions occurring with an incidence of greater than 2% in the 30 men treated with Follistim are listed in Table 4.
| System Organ Class/Adverse Reactions | Follistim Treatment N=30 n (%) |
|---|---|
| Nervous system disorders | |
| Headache | 2 (6.7) |
| General disorders and administration site disorders | |
| Injection site reaction | 2 (6.7) |
| Injection site pain | 2 (6.7) |
| Skin and cutaneous tissue disorders | |
| Acne | 2 (6.7) |
| Rash | 1 (3.3) |
| Reproductive system and breast disorders | |
| Gynecomastia | 1 (3.3) |
| Neoplasms benign, malignant and unspecified | |
| Dermoid cyst | 1 (3.3) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Follistim and/or Follistim AQ Cartridge. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Vascular disorders
Thromboembolism [see Warnings and Precautions (5.3)]
7 DRUG INTERACTIONS
No drug-drug interaction studies have been performed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X: Follistim AQ Cartridge should not be used during pregnancy [see Contraindications (4)]
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in the nursing infant from Follistim AQ Cartridge, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of Follistim did not include subjects aged 65 and over.
10 OVERDOSAGE
Aside from the possibility of Ovarian Hyperstimulation Syndrome [see Warnings and Precautions (5.2, 5.3)] and multiple gestations [see Warnings and Precautions (5.5)], there is no additional information concerning the consequences of acute overdosage with Follistim AQ Cartridge.
11 DESCRIPTION
Follistim AQ Cartridge contains human follicle-stimulating hormone (hFSH), a glycoprotein hormone which is manufactured by recombinant DNA (rDNA) technology. The active drug substance, follitropin beta, has a dimeric structure containing two glycoprotein subunits (alpha and beta). Both the 92 amino acid alpha-chain and the 111 amino acid beta-chain have complex heterogeneous structures arising from two N-linked oligosaccharide chains. Follitropin beta is synthesized in a Chinese hamster ovary (CHO) cell line that has been transfected with a plasmid containing the two subunit DNA sequences encoding for hFSH. The purification process results in a highly purified preparation with a consistent hFSH isoform profile and high specific activity [as determined by the Ph. Eur. test for FSH in vivo bioactivity and on the basis of the molar extinction coefficient at 277 nm (εs:mg-1cm-1) = 1.066].
The biological activity is determined by measuring the increase in ovary weight in female rats. The intrinsic luteinizing hormone (LH) activity in follitropin beta is less than 1 international unit per 40,000 international units FSH. The compound is considered to contain no LH activity.
The amino acid sequence and tertiary structure of the product are indistinguishable from that of hFSH of urinary source. Also, based on available data derived from physico-chemical tests and bioassay, follitropin beta and follitropin alfa, another recombinant follicle-stimulating hormone product, are indistinguishable.
Follistim AQ Cartridge is a ready for use, prefilled with solution, disposable cartridge containing either 175 IU of follitropin beta in 0.210 mL (833 IU/mL), 350 IU in 0.420 mL (833 IU/mL), 650 IU in 0.780 mL (833 IU/mL) or 975 IU in 1.170 mL (833 IU/mL) of aqueous solution for multiple dose use, with a maximal deliverable dose of either 150 IU, 300 IU, 600 IU or 900 IU, respectively. Inactive ingredients in the cartridges include: benzyl alcohol NF 10 mg/mL; L-methionine USP 0.5 mg/mL; polysorbate 20 NF 0.2 mg/mL; sodium citrate (dihydrate) USP 14.7 mg/mL; sucrose NF 50 mg/mL; and water for injection USP. Hydrochloric acid NF and/or sodium hydroxide NF are used to adjust the pH to 7.
Follistim AQ Cartridge is for use only with the Follistim Pen, which features an adjustable dosing system for administering the drug in a microvolume of solution. The Follistim Pen with Follistim AQ Cartridge is intended for SUBCUTANEOUS USE ONLY. The recombinant protein in Follistim AQ Cartridge has been standardized for FSH in vivo bioactivity in terms of the WHO International Standard for Follicle Stimulating Hormone (FSH) Recombinant, Human for Bioassay (code 92/642), issued by the World Health Organization Expert Committee on Biological Standardization (1995). Under current storage conditions, Follistim AQ may contain up to 11% of oxidized follitropin beta.
In clinical trials with Follistim, serum antibodies to FSH or anti-CHO cell derived proteins were not detected in any of the treated patients after exposure to Follistim for up to three cycles.
Therapeutic Class: Infertility.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Women:
Follicle-stimulating hormone (FSH), the active component in Follistim AQ Cartridge, is required for normal follicular growth, maturation, and gonadal steroid production.
In women, the level of FSH is critical for the onset and duration of follicular development, and consequently for the timing and number of follicles reaching maturity. Follistim AQ Cartridge stimulates ovarian follicular growth in women who do not have primary ovarian failure. In order to effect the final phase of follicle maturation, resumption of meiosis and rupture of the follicle in the absence of an endogenous LH surge, human chorionic gonadotropin (hCG) must be given following treatment with Follistim AQ Cartridge when patient monitoring indicates appropriate follicular development parameters have been reached.
Men:
Follistim when administered with hCG stimulates spermatogenesis in men with hypogonadotropic hypogonadism. FSH, the active component of Follistim, is the pituitary hormone responsible for spermatogenesis.
12.3 Pharmacokinetics
Pharmacokinetic parameters for Follistim AQ Cartridge were evaluated in an open-labeled, single-center, randomized study in 20 healthy women. Serum FSH values from a single subcutaneous injection of reconstituted Follistim lyophilized powder administered by conventional syringe were compared to those values following a single subcutaneous injection of Follistim AQ Cartridge administered with the Follistim Pen injector. Administration of follitropin beta with the Follistim Pen resulted an 18% increase in AUC0–∞ and Cmax. The 18% difference in serum FSH concentrations resulting from administration of the two formulations was due to differences between the anticipated and actual volume delivered with the conventional syringe. The pharmacokinetic parameters for Follistim AQ Cartridge are as follows:
| AUC0–∞
(IU/L*h) | Cmax
(IU/L) | tmax
(h) | t1/2
(h) | CLapp
(L/h/kg) |
|
|---|---|---|---|---|---|
| AUC0–∞ Area under the curve | |||||
| Cmax Maximum concentration | |||||
| tmax Time to maximum concentration | |||||
| t1/2 Elimination half-life | |||||
| CLapp Clearance | |||||
| Follistim AQ Cartridge | 215.1 (45.8) | 3.4 (0.7) | 12.9 (6.2) | 33.4 (4.2) | 0.01 (0.003) |
Absorption:
Women:
The bioavailability of Follistim following subcutaneous and intramuscular administration was investigated in healthy, pituitary-suppressed, women given a single 300 international units dose. In these women, the area under the curve (AUC), expressed as the mean ± SD, was equivalent between the subcutaneous (455.6 ± 141.4 IU*h/L) and intramuscular (445.7 ± 135.7 IU*h/L) routes of administration. However, equivalence could not be established with respect to the peak serum FSH levels (Cmax). The Cmax achieved after subcutaneous administration and intramuscular administration was 5.41 ± 0.72 international units/L and 6.86 ± 2.90 international units/L, respectively. After subcutaneous or intramuscular injection the apparent dose absorbed was 77.8% and 76.4%, respectively.
The pharmacokinetics and pharmacodynamics of a single, intramuscular dose (300 international units) of Follistim were also investigated in a group (n=8) of gonadotropin-deficient, but otherwise healthy women. In these women, FSH (mean ± SD) AUC was 339 ± 105 international units*h/L, Cmax was 4.3 ± 1.7 international units/L. Cmax occurred at approximately 27 ± 5.4 hours after intramuscular administration.
A multiple dose, dose proportionality, pharmacokinetic study of Follistim was completed in healthy, pituitary-suppressed, female subjects given subcutaneous doses of 75, 150, or 225 international units for 7 days. Steady-state blood concentrations of FSH were reached with all doses after 5 days of treatment based on the trough concentrations of FSH just prior to dosing (Ctrough). Peak blood concentrations with the 75, 150, and 225 international units dose were 4.30 ± 0.60 international units/L, 8.51 ± 1.16 international units/L and 13.92 ± 1.81 international units/L, respectively.
Men:
No PK studies were conducted using Follistim AQ Cartridge in men. Exposures of follitropin beta from Follistim AQ Cartridge and Follistim are expected to be equivalent after adjusting for the 18% difference in dose [see Dosage and Administration (2)].
Serum levels of FSH were measured in a clinical study that compared the effects of two different dosing schedules of Follistim (150 international units three times a week or 225 international units twice a week) administered by subcutaneous injection concurrently with chorionic gonadotropin for induction of spermatogenesis in hypogonadotropic hypogonadal men. Administration of Follistim was started at week 17. Mean serum trough concentrations of FSH remained fairly constant over the treatment period. At the end of treatment (week 64), the mean serum trough concentrations of FSH were 2.09 international units/L in the 150 international units group and 3.22 international units/L in the 225 international units group. Serum trough concentrations of FSH measured prior to the first Follistim injection on the Mondays of active treatment period (weeks 17 to 64) and one week after the end of treatment period are presented in Figure 1.
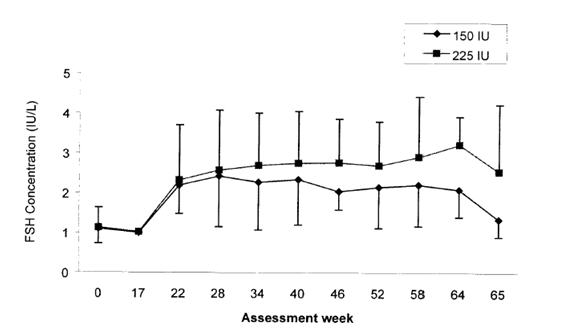
FIGURE 1: Mean (SD) Serum Trough Concentrations of FSH in Men Following Subcutaneous Administration of Follistim Using Two Different Dosing Schedules (150 International Units Three Times a Week or 225 International Units Twice a Week)
Distribution:
The volume of distribution of Follistim in healthy, pituitary-suppressed, women following intravenous administration of a 300 international units dose was approximately 8 L.
Metabolism:
The recombinant FSH in Follistim AQ Cartridge is biochemically very similar to urinary FSH and it is therefore anticipated that it is metabolized in the same manner.
Elimination:
The elimination half-life (t1/2) following a single subcutaneous injection of 150 IU of Follistim AQ Cartridge in women was 33.4 (4.2) hours. The clearance was 0.01 (0.003) L/h/kg.
Use in Specific Populations:
Body weight: The effect of body weight on the pharmacokinetics of Follistim was evaluated in a group of European and Japanese women who were significantly different in terms of body weight. The European women had a body weight of (mean ± SD) 67.4 ± 13.5 kg and the Japanese subjects were 46.8 ± 11.6 kg. Following a single intramuscular dose of 300 international units of Follistim, the AUC was significantly smaller in European women (339 ± 105 international units*h/L) than in Japanese women (544 ± 201 international units*h/L). However, clearance per kg of body weight was essentially the same for the respective groups (0.014 and 0.013 L/hr/kg).
Geriatric Use: The pharmacokinetics of Follistim has not been studied in geriatric subjects.
Pediatric Use: The pharmacokinetics of Follistim has not been studied in pediatric subjects.
Renal Impairment: The effect of renal impairment on the pharmacokinetics of Follistim has not been studied.
Hepatic Impairment: The effect of hepatic impairment on the pharmacokinetics of Follistim has not been studied.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term toxicity studies in animals have not been performed with Follistim to evaluate the carcinogenic potential of the drug. Follistim was not mutagenic in the Ames test using S. typhimurium and E. coli tester strains and did not produce chromosomal aberrations in an in vitro assay using human lymphocytes.
14 CLINICAL STUDIES
14.1 Ovulation Induction
The efficacy of Follistim for Ovulation Induction was evaluated in a randomized, assessor-blind, parallel-group comparative, multicenter safety and efficacy study of 172 chronic anovulatory women (105 subjects on Follistim) who had previously failed to ovulate and/or conceive during clomiphene citrate treatment. The study results for ovulation rates are summarized in Table 6 and those for pregnancy rates are summarized in Table 7.
| Cycle | Follistim (n=105) |
|---|---|
| First treatment cycle | 72% |
| Second treatment cycle | 82% |
| Third treatment cycle | 85% |
| Cycle | Follistim (n=105) |
|---|---|
| First treatment cycle | 14% |
| Second treatment cycle | 19% |
| Third treatment cycle | 23% |
14.2 Assisted Reproductive Technology (ART)
The efficacy of Follistim as part of an Assisted Reproductive Technology (ART) program was established in three studies, two of which are described below.
Follistim was evaluated in a randomized, assessor-blind, parallel-group, comparative, multicenter safety and efficacy study of 981 healthy normal ovulatory infertile women (mean age 32) treated for multiple cycles with in vitro fertilization and controlled ovarian stimulation with Follistim (n=585) or urofollitropin (n=396) after pituitary suppression with a GnRH agonist. The first cycle results with Follistim are summarized in Table 8.
| Parameter | Follistim (n=585) |
|---|---|
|
|
| Total number of oocytes recovered | 10.9 |
| Ongoing† pregnancy rate/attempt‡ | 22.2% |
| Ongoing† pregnancy rate/transfer‡, § | 26.0% |
Follistim was also evaluated in a randomized, assessor-blind, parallel-group, comparative, single center safety and efficacy study in 89 infertile healthy normal ovulatory women (mean age 32) treated for one cycle with in vitro fertilization and controlled ovarian stimulation with Follistim (n=54) or menotropins (n=35) without pituitary suppression with a GnRH agonist. The results with Follistim are summarized in Table 9.
| Parameter | Follistim (n=54) |
|---|---|
|
|
| Total number of oocytes recovered | 9.9 |
| Ongoing† pregnancy rate/attempt‡ | 22.2% |
| Ongoing† pregnancy rate/transfer‡, § | 30.8% |
14.3 Induction of Spermatogenesis
The safety and efficacy of Follistim administered by subcutaneous injection concomitantly chorionic gonadotropin for injection (hCG) has been examined in a multicenter, open-label, non-comparator clinical study for induction of spermatogenesis in hypogonadotropic hypogonadal men. The study compared the effects of two different Follistim dosing schedules on semen parameters and serum levels of follicle stimulating hormone (FSH). The multicenter study involved a 16-week pretreatment phase with hCG at a dosage of 1500 international units twice a week to normalize serum testosterone levels. If serum testosterone levels did not normalize after 8 weeks of hCG treatment, the hCG dose could have been increased to 3000 international units twice a week. This phase was followed by a 48-week treatment phase. Men who were still azoospermic after the pretreatment phase were randomized to receive either 225 international units Follistim together with 1500 international units hCG twice a week or 150 international units Follistim three times a week together with 1500 international units hCG twice weekly. Men who required 3000 international units of hCG twice a week in the pretreatment phase were continued on that dosage during the treatment phase. The mean age of patients in both treatment groups was approximately 30 years (range 18 to 47 years). At baseline, mean left and right testis volumes were 4.61 ± 2.94 mL and 4.57 ± 3.00 mL, respectively, in the group receiving three weekly injections of Follistim. For the group receiving two weekly injections of Follistim, the mean left and right testis volumes were 6.54 ± 2.45 mL and 7.21 ± 2.94 mL, respectively, at baseline. The primary efficacy endpoint was the percentage of patients with a mean sperm density of ≥1 × 106/mL on their last two treatment assessments. The outcomes of treatment in the 30 men enrolled in the treatment phase are summarized in Table 10.
| Follistim 150 international units three times a week (n=15) | Follistim 225 international units twice a week (n=15) | Overall (n=30) |
||||
|---|---|---|---|---|---|---|
| Sperm Density of ≥106/mL | n | % | n | % | n | % |
| Yes | 6 | 40 | 7 | 47 | 13 | 43 |
| No | 9 | 60 | 8 | 53 | 17 | 57 |
Overall, the median time to reach a sperm concentration of 106 per mL was 165 days (range 25 to 327 days) in patients who demonstrated a sperm concentration of at least 106 per mL. The median time to reach a sperm concentration of at least 106 per mL was 186 days (range 25 to 327 days) for the 150 international units group and 141 days (range 43 to 204 days) for the 225 international units group. No pregnancy data were collected during the trial.
The local tolerance data were comparable between the two treatment groups. The mean percentage of days without pain calculated for all subjects in the treatment period was 91.3% for patients in the 150 international units (three times a week) and 76.0% for patients in the 225 international units (two times a week) Follistim treatment groups. In the 225 international units (twice per week) group, local symptoms judged as severe by the investigator were: itching in 1 patient (7%), pain in 2 patients (13%), bruising in 2 patients (13%), swelling in 2 patients (13%), and redness in 1 patient (7%). In the 150 international units (three times per week) group, 1 event in 1 patient (bruising, 7%) was judged as severe. No patient discontinued treatment due to injection site reaction or injection site pain.
16 HOW SUPPLIED/STORAGE AND HANDLING
Follistim AQ Cartridge is supplied in a box containing disposable, 29 gauge, ultra-fine, 1/2-inch, sterile BD Micro-Fine™ Pen Needles (for use with Follistim Pen available separately) and one disposable, blister packed, prefilled 1.5 mL colorless glass cartridge, with grey rubber piston and an aluminum crimp-cap with black rubber inlay and in the following presentations:
- NDC 0052-0303-01 Follistim AQ Cartridge 175 international units per 0.210 mL (delivering 150 international units) with orange crimp-caps and 3 BD Micro-Fine Pen Needles
- NDC 0052-0313-01 Follistim AQ Cartridge 350 international units per 0.420 mL (delivering 300 international units) with silver crimp-caps and 5 BD Micro-Fine Pen Needles
- NDC 0052-0316-01 Follistim AQ Cartridge 650 international units per 0.780 mL (delivering 600 international units) with gold crimp-caps and 7 BD Micro-Fine Pen Needles
- NDC 0052-0326-01 Follistim AQ Cartridge 975 international units per 1.170 mL (delivering 900 international units) with blue crimp-caps and 10 BD Micro-Fine Pen Needles
Store refrigerated 2-8°C (36-46°F) until dispensed. Upon dispensing, the product may be stored by the patient at 2-8°C (36-46°F) until the expiration date, or at 25°C (77°F) for 3 months or until expiration date, whichever occurs first. Once the rubber inlay of the Follistim AQ Cartridge has been pierced by a needle, the product can only be stored for a maximum of 28 days at 2-25°C (36-77°F). Protect from light. Do not freeze.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling
17.1 Dosing and Use of Follistim AQ Cartridge with Pen
Instruct women and men on the correct usage and dosing of Follistim AQ Cartridge in conjunction with the Follistim Pen. Make sure that individuals who have used other gonadotropin products delivered by a syringe are aware of differences arising from use of the pen. Women and men should read and follow all instructions in the Follistim Pen "Instructions for Use" Manual prior to administration of Follistim AQ Cartridge.
Advise women and men of the number of doses which can be extracted from the full unused Follistim AQ Cartridge that you have prescribed.
17.2 Therapy Duration and Necessary Monitoring in Women and Men Undergoing Treatment
Prior to beginning therapy with Follistim AQ Cartridge, inform women and men about the time commitment and monitoring procedures necessary to undergo treatment [see Dosage and Administration (2), Warnings and Precautions (5.10)].
17.3 Instructions on a Missed Dose
Inform women and men that if they miss or forget to take a dose of Follistim AQ Cartridge, the next dose should not be doubled and they should call the healthcare provider for further dosing instructions.
17.4 Ovarian Hyperstimulation Syndrome
Inform women regarding the risks with use of Follistim AQ Cartridge of Ovarian Hyperstimulation Syndrome [see Warnings and Precautions (5.2)] and associated symptoms including lung and blood vessel problems [see Warnings and Precautions (5.3)] and ovarian torsion [see Warnings and Precautions (5.4)].
17.5 Multi-fetal Gestation and Birth
Inform women regarding the risk of multi-fetal gestations with the use of Follistim AQ Cartridge [see Warnings and Precautions (5.5)].
Manufactured for Organon USA Inc., Roseland, NJ 07068
by Vetter Pharma-Fertigung GmbH & Co. KG, Ravensburg, Germany
and packaged by Organon (Ireland) Ltd., Swords, Co. Dublin, Ireland
BD, BD Logo and BD Micro-Fine are trademarks of Becton, Dickinson and Company
U.S. Patent Nos. 5,767,251; 5,929,028; 7,446,090 and 7,563,763.
Copyright © 2004, 2010 N.V. Organon. All rights reserved.
Rev. 9/10
B-33554419
34306508T
PATIENT INFORMATION
Follistim® AQ Cartridge
(follitropin beta injection)
Read the Patient Information that comes with Follistim® AQ Cartridge before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is Follistim AQ Cartridge?
Follistim AQ is a prescription medicine that contains follicle-stimulating hormone (FSH). The medicine is taken with the Follistim Pen®.
Follistim AQ Cartridge is used:
In women:
- to help healthy ovaries to develop (mature) and release eggs
- as part of an Assisted Reproductive Technology (ART) program to help the ovaries produce more mature eggs
In men:
- to help bring about the production and development of sperm
Who should not take Follistim AQ Cartridge?
Do not take Follistim AQ Cartridge if you are a Woman or Man who:
- is allergic to recombinant human FSH products
- has a high level of FSH in your blood indicating that your ovaries (women only) or testes (men only) may be permanently damaged and do not work at all
- has uncontrolled thyroid, pituitary, or adrenal gland problems
- is allergic to streptomycin or neomycin (types of antibiotics)
- has a tumor of the hypothalamus, pituitary gland, breast, uterus (women only), ovary (women only), or testis (men only)
Do not take Follistim AQ Cartridge if you are a Woman who:
- is pregnant or think you may be pregnant
- has heavy or irregular vaginal bleeding and the cause is not known
- has ovarian cysts or enlarged ovaries, not due to polycystic ovary syndrome (PCOS)
Talk to your healthcare provider before taking this medicine if you have any of the conditions listed above.
What should I tell my healthcare provider before taking Follistim AQ Cartridge?
Before you take Follistim AQ, tell your healthcare provider if you:
- have an increased risk of blood clots (thrombosis)
- have ever had a blood clot (thrombosis), or anyone in your immediate family has ever had a blood clot (thrombosis)
- had stomach (abdominal) surgery
- had twisting of your ovary (ovarian torsion)
- had or have a cyst in your ovary
- have polycystic ovary disease
- have any other medical conditions
- are breastfeeding or plan to breastfeed. It is not known if the medicine in Follistim AQ Cartridge passes into your breast milk. You and your healthcare provider should decide if you will take Follistim AQ Cartridge or breastfeed. You should not do both
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them and show your healthcare provider and pharmacist when you get a new medicine.
How should I use Follistim AQ Cartridge?
- Be sure that you read, understand, and follow the "Patient Instructions for Use" that come with Follistim AQ Cartridge
- Use Follistim AQ Cartridge exactly as your healthcare provider tells you to
- Your healthcare provider will tell you how much Follistim AQ Cartridge to use, how to inject it, and how often it should be injected
- Do not inject Follistim AQ Cartridge at home until your healthcare provider has taught you the right way to put the cartridge and pen device together and to inject yourself
- Do not mix any other medicines into the Cartridge
- Do not change your dose of Follistim AQ Cartridge unless your healthcare provider tells you to
- Call your healthcare provider immediately if you use too much Follistim AQ Cartridge
- If you miss or forget to take a dose, do not double your next dose. Ask your healthcare provider for instructions
- Use Follistim AQ Cartridge only with the Follistim Pen
- Do not use the Follistim Pen if you are blind or visually impaired unless you have assistance from an individual with good vision who is trained in the right way to use the pen
- Do not re-use the BD Micro-Fine™ Pen Needle
- Your healthcare provider will do blood and urine hormone tests while you are taking Follistim AQ Cartridge. Make sure you follow-up with your healthcare provider to have your blood and urine tested when told to do so
Women:
- Your healthcare provider may do ultrasound scans of your ovaries. Make sure you follow-up with your healthcare provider to have your ultrasound scans
Men:
- Your healthcare provider may test your semen while you are taking Follistim AQ Cartridge. Make sure you follow-up with your healthcare provider to give a semen sample for testing
What are the possible side effects of Follistim AQ Cartridge?
Follistim AQ Cartridge may cause serious side effects.
Serious side effects in women include:
- Ovarian enlargement
-
Ovarian hyperstimulation syndrome (OHSS). OHSS is a serious medical problem that can happen when the ovaries are over stimulated. In rare cases it has caused death. OHSS causes fluid to build up suddenly in your stomach and chest areas and can cause blood clots to form. Call you healthcare provider right away if you have:
- pain in your lower stomach area
- nausea
- vomiting
- weight gain
- diarrhea
- decreased urine output
- trouble breathing
- Lung problems. Follistim AQ Cartridge can cause you to have fluid in your lungs (atelectasis) and trouble breathing (acute respiratory distress syndrome).
-
Blood clots. Follistim AQ Cartridge may increase your chance of having blood clots in your blood vessels. Blood clots can cause:
- blood vessel problems (thrombophlebitis)
- stroke
- loss of your arm or leg
- blood clot in your lungs (pulmonary embolus)
- heart attack
- Ovarian torsion. Follistim AQ Cartridge may increase the chance of twisting of the ovaries in women with certain conditions such as OHSS, pregnancy and previous abdominal surgery. Twisting of the ovary could cause the blood flow to the ovary to be cut off
- Pregnancy and birth of multiple babies. Having a pregnancy with more than one baby at a time increases the health risk for you and your babies. Discuss your chances of multiple births with your healthcare provider
- Birth defects. A woman's age, certain sperm problems, genetic background of both parents and a pregnancy with multiple babies can increase the chance that your baby might have birth defects
- Ectopic pregnancy (pregnancy outside of the womb). The chance of a pregnancy outside of the womb is increased in women with damaged fallopian tubes
- Miscarriage. The chance of loss of an early pregnancy may be increased in women who have difficulty with becoming pregnant at all
The most common side effects of Follistim AQ Cartridge include:
In women:
- Cyst in the ovary
- stomach pain
In Men:
- headache
- pain at the injection site
- bruising, swelling or redness at the injection site
- breast enlargement
- acne
These are not all the possible side effects of Follistim AQ Cartridge. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider immediately if you get worsening or strong abdominal pain. Also, call your healthcare provider immediately if this happens some days after the last injection has been given.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Follistim AQ Cartridge?
- Store Follistim AQ Cartridge in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date
- Follistim AQ can be stored at or below 77°F (25°C) for 3 months or until the expiration date, whichever comes first. Once the rubber inlay of the Follistim AQ Cartridge has been pierced by a needle, the product may be stored only for a maximum of 28 days at 36°F to 77°F (2°C to 25°C).
- Keep Follistim AQ Cartridge away from light
- Do not freeze
Keep Follistim AQ Cartridge and all medicines out of the reach of children.
General information about Follistim AQ Cartridge
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Follistim AQ for a condition for which it was not prescribed. Do not give Follistim AQ Cartridge to other people, even if they have the same condition that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Follistim AQ Cartridge. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for more information about Follistim AQ Cartridge that is written for healthcare professionals.
For more information, go to www.follistim.com or call 1-866-836-5633.
What are the ingredients in Follistim AQ Cartridge?
Active ingredient: follitropin beta
Inactive ingredients: sucrose, sodium citrate, benzyl alcohol NF-10 mg/mL, L-methionine, polysorbate 20, water for injection, hydrochloric acid, and/or sodium hydroxide.
Manufactured for Organon USA Inc.
Roseland, NJ 07068
by Vetter Pharma-Fertigung GmbH & Co. KG
Ravensburg, Germany
and packaged by Organon (Ireland) Ltd.
Swords, Co. Dublin, Ireland
BD, BD Logo and BD Micro-Fine are trademarks of Becton, Dickinson and Company
U.S. Patent Nos. 5,767,251; 5,929,028; 7,446,090 and 7,563,763.
Copyright © 2004, 2010 N.V. Organon. All rights reserved.
Rev. 9/10
PATIENT INSTRUCTIONS FOR USE
Follistim® (Fol´-lis-tim) AQ Cartridge
(follitropin beta injection)
Read the Patient Instructions for Use that comes with Follistim® AQ Cartridge before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
A. Getting Ready
- Follistim Pen is not recommended for the blind or visually impaired user without the assistance of an individual with good vision, trained in the proper use of the injection device.
- Learn about all of the parts of the Follistim Pen (See Figure 1), Follistim AQ Cartridge (Figure 2) and the BD Micro-Fine™ Pen Needle (Figure 3). You will need to recognize these parts to follow the directions
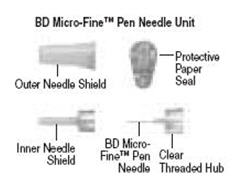
Figure 3. Parts of BD Micro-Fine Pen Needle Unit
- Remove the Cartridge out of the refrigerator
- Injecting cold drug is likely to cause discomfort. Therefore, it is recommended you allow the drug to reach room temperature before taking the injection.
- Check the liquid in the cartridge. It should appear clear and colorless. If the solution is not clear and colorless or has particles in it, do not use it
-
Gather the supplies you will need for your injection. You will need:
- a clean dry surface
- alcohol
- cotton balls or alcohol pads
- sterile gauze
- a puncture-proof container to throw away the used syringe and needle
- Wash your hands with soap and water and dry them before you use Follistim Pen or when you replace the cartridge.
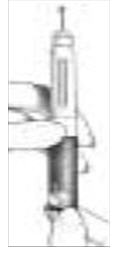
B. Loading the Follistim Pen with the Follistim AQ Cartridge
| |
| |
| |
| |
| |
| |
|
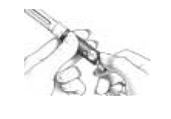
C. Preparing the Injection Site
|
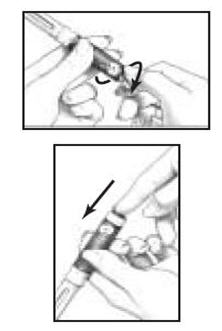
D. Dialing the Dose Before You Give the Injection
| |
|
|
| |
|
|
| |
- With a partially used cartridge, to give yourself another dose of medicine you will need to attach a new BD Micro-Fine Pen Needle and look for a droplet forming at the tip of the needle (See Figure 15 above). If you see a droplet, then you can dial in your dose
If no droplet:
- Dial the Dosage Knob until you hear one click. With the needle pointing upwards, push in the Injection Button
- Look for a droplet at the tip of the needle. If you see the droplet, then you can dial in your dose
- Your Follistim AQ Cartridge should be one of the following:
- Orange Metal Cap – 150 international units
- Silver Metal Cap – 300 international units
- Gold Metal Cap – 600 international units
- Blue Metal Cap – 900 international units
If you did not understand that you should have one of the cartridges above, please contact your healthcare provider
|
- If by mistake you dial past the correct number, do not try to turn the Dosage Knob backward to fix the mistake. Continue to turn the Dosage Knob in the same direction past the 450 IU mark, as far as it will turn. The Dosage Scale must move freely. Push the Injection Button in all the way. See Figure 17. Start to dial again starting from "0" upwards. By following these directions, you will not lose any medicine from the Follistim AQ Cartridge (See "Checking the Medicine Level Remaining")
- If you turn the Dosage Knob backward to correct the mistake, it will not damage the Pen, but you will lose some medication from the Follistim AQ Cartridge
- Never dial your dose or try to correct a dialing mistake when the needle is still in your skin as this may result in your receiving an incorrect dose.
- If your prescribed dose exceeds the deliverable dose of Follistim Pen or exceeds the amount remaining in the cartridge, you will need to give yourself more than one injection
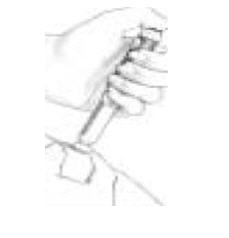
E. Giving Yourself an Injection
|
If the injection button does not push in all the way, and the number in the Dosage Window does not read "0", it means there is not enough medication left in the cartridge to complete your prescribed dose. The number in the Dosage Window will give you the amount of medicine needed to complete your dose. Write this number down. This will be the number you dial for the completion of your dose. Start over with a new Follistim AQ Cartridge and a new needle and follow all the instructions up to this step. Make sure you choose a different injection site to complete your dose of Follistim AQ Cartridge.
| |
| |
|
|
| |
| |
F. Checking the Medicine Level Remaining
For women and men:
Your healthcare provider should advise you of the number of prescribed doses which can be extracted from the full unused Follistim AQ Cartridge.
- Do not use the cartridge beyond the advised number of doses. Otherwise, you will run the risk that there will not be enough volume of drug for your prescribed dose.
For women only:
- Keep a Follistim Pen Treatment Diary as follows:
- Record the Follistim AQ Cartridge content on Day 1. This will either be 150, 300, 600 or 900 international units depending on what your healthcare provider has prescribed for you.
- Record the dose you have been prescribed for your injection.
- Subtract your Day 1 dose from the Follistim AQ Cartridge content (150, 300, 600 or 900 international units) (See example – Figure 23). This will give you the remaining Follistim AQ Cartridge content after the day 1 dose is taken.
- Place the number determined as the content after Day 1 (see number 3) in the box as the Follistim AQ Cartridge content available for Day 2 (See example – Figure 23).
- Subtract your Day 2 dose from the Follistim AQ Cartridge content you recorded in Step 4. This will give you the remaining Follistim AQ Cartridge content after Day 2. Record this (See example – Figure 23.
- Repeat the steps to determine the Follistim AQ Cartridge content available and Follistim AQ Cartridge remaining for each day of use.
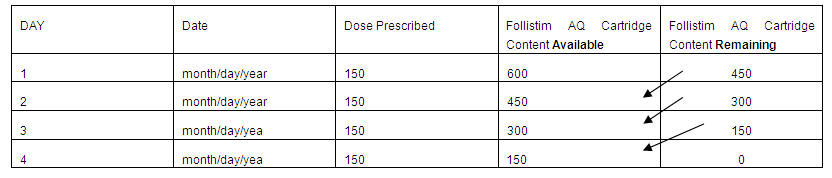 |
| Figure 23. Example of Treatment Diary Starting with a 600 International Unit Cartridge |
- If you do not know if there is not enough medicine left in the Follistim AQ Cartridge for your next prescribed dose, see section 'IF THERE IS NOT ENOUGH FOLLISTIM AQ IN THE CARTRIDGE'.
G. IF THERE IS NOT ENOUGH FOLLISTIM AQ IN THE CARTRIDGE
- If you realize before you inject that you do not have enough medicine remaining in your Follistim AQ Cartridge for your complete dose, follow either Option 1 or Option 2, but not both
- Option #1:
- Dial your dose and inject the remaining content in the Follistim AQ Cartridge. The Dosage Knob Injection Button will not push in all the way (do not try to force down the button) and the Dosage Window number will not read "0" but will read the number of units you will need to complete your prescribed dose
- Write down the number of units needed to complete your dose
- Remove the needle and dispose of it properly as (see "How Do I Throw Away Used Cartridges and Needles?")
- Using the Dosage Knob, reset the Dial Window to "0" by turning the Dosage Knob past the 450 IU mark as far as it will turn and push the Injection Button in all the way
- Before attempting to replace a Follistim AQ Cartridge, be sure that a BD Micro-Fine Pen Needle is not attached to the Follistim Pen
- Insert a new cartridge into the Follistim Pen and attach a new BD Micro-Fine needle
- Dial to the number of units you have written down to complete your prescribed dose.
- Prepare a different injection site and inject the remaining drug to complete your dose (See "Giving Yourself an Injection")
- Option #2.
- Remove the Follistim AQ Cartridge.
- Start over with a new Follistim AQ Cartridge and Insert into the Follistim Pen
- Follow the instructions for "Dialing the Dose" and "Giving Yourself an Injection"
- Option #1:
- If you realize after you have inserted the needle at the injection site that you do not have enough medicine remaining in your Follistim AQ Cartridge for your complete dose:
- Inject the remaining content in the Follistim AQ Cartridge. The Injection Button will not push in all the way and the number in the Dosage Window will not read "0" but will read the number of units you will need to complete your prescribed dose.
- Wait 5 seconds before withdrawing the needle from your skin and gently apply pressure to the injection site with an alcohol pad.
- Dispose of the used needle (See "How Do I Throw Away Used Cartridges and Needles?").
- Write down the number of units needed to complete your dose.
- Using the Dosage Knob, reset the Dial Window to "0" by turning the Dosage Knob past the 450 IU mark as far as it will turn and push the Injection Button in all the way.
- Insert a new cartridge into the Follistim Pen and attach a new BD Micro-Fine needle.
- Dial to the number you have recorded to complete your prescribed dose.
- Prepare a different injection site and inject the remaining drug to complete your dose (See "Giving Yourself an Injection").
H. How to Solve Problems with Follistim AQ Cartridge and Follistim Pen
- If you have problems with using the Follistim AQ Cartridge and the Follistim Pen, see the following chart. If you still have problems after following the chart or if your problem is not on the chart, contact your healthcare provider.
| PROBLEM | POSSIBLE CAUSES | WHAT TO DO |
|---|---|---|
| The Pen Body will not screw tightly into the Cartridge Holder. | Is something in the way? | Take out the Follistim cartridge and check the Cartridge Holder to see if anything is in the way. Follow the instructions in this pamphlet to Screw the Pen body fully onto the Cartridge Holder. |
| No drug is coming out while checking the flow. | The Cartridge Holder and the Pen Body are not properly screwed together. | Remove the current needle; tighten the Pen Body to the Cartridge Holder ensuring the arrow on the Cartridge Holder is pointing to the middle of the yellow alignment mark on the blue Pen Body. Attach a new needle to the Pen. Recheck the flow as follows: a. Dial the Dosage Knob until you hear one click. With needle pointing upwards, push in the Injection Button. b. Look for a droplet at the tip of the needle. |
| Is the Follistim cartridge empty? | Change to a new cartridge. | |
| Has the needle been properly attached to the Follistim Pen? | Remove needle and replace with a new one, ensuring that the needle is screwed on tightly to the Pen. Recheck the flow as follows: a. Dial the Dosage Knob until you hear one click. With needle pointing upwards, push in the Injection Button. b. Look for a droplet at the tip of the needle. |
|
| You are concerned that you can turn the Dosage Knob to the next number without clicking and the injection button spins freely. | This is not a problem. | The system is in the reset mode. The Injection Button and Dosage Knob must be pushed all the way down to '0' to re-engage the mechanism and the correct dose can now be set. A click will be heard for each setting in the viewing window. |
| The Dosage Knob does not go back to '0' while you are injecting. | Is the Follistim cartridge empty? | Change to a new cartridge. |
| Is the needle blocked? | a. Remove the needle from the skin and dispose of safely. b. Check the Dosage Window and note how much remaining drug to inject. c. Attach a new needle. Recheck the flow as follows: a. Dial the Dosage Knob until you hear one click. With needle pointing upwards, push in the Injection Button. b. Look for a droplet at the tip of the needle. c. Dial remaining dose. |
|
| Some of the drug is dripping out of the needle when you withdraw it from your skin. | Did you take the needle out of your skin before waiting 5 seconds as directed in Step 15? | If this happens you should inform your doctor. To avoid this problem again, you should always wait 5 seconds after you push the Injection Button before you withdraw the needle from your skin. |
| The needle is left on the Follistim Pen. | Have you missed any of the instructions? | Dispose of the needle in a properly secured container as instructed by your doctor. Change to a new Follistim cartridge and a new needle. |
| After your last injection, a remaining volume may be left in the cartridge in addition to the normal quantity of drug dispensed. | The cartridge contains extra volume for checking the drug flow. | This is not a problem. |
| You cannot get the cartridge out of the Follistim Pen. | Is the needle attached? | Remove the needle from the Follistim Pen and dispose of properly. (Unscrew the Cartridge Holder from the Pen Body and take out the cartridge.) |
| You are not sure how much drug is left in the cartridge and you do not want to start an injection and then find out that there is not enough drug. | Have you kept good records of your doses? | In case of any doubt, you should load a new, unused Follistim cartridge into the Follistim Pen. See "IF THERE IS NOT ENOUGH FOLLISTIM AQ IN THE CARTRIDGE"
To avoid this problem again, you should record your injections (Women should use a treatment diary). |
Important: If you have a question, always mention the Lot number of your Follistim Pen as printed on the pen body. If you have a complaint, please do not discard any product or packaging.
For questions on information contained in this leaflet, call 1-866-836-5633.
www.follistim.com
How Do I Throw Away Used Cartridges and Needles?
- Check with your healthcare provider or pharmacist for instructions about the right way to throw away used cartridges and needles. There may be special local or state laws about how to throw away used syringes and needles
- Do not throw away used cartridges and needles in the household trash and do not recycle them
- Put used and empty cartridges and needles in a closeable, puncture-resistant container. You may use a sharps container (such as a red bio-hazard container), a hard plastic container with a screw-on cap (such as an empty detergent bottle) or in a metal container with a plastic lid, (such as a coffee can)
- When the container is full, tape around the cap or lid to make sure the cap or lid does not come off
- When your injection is given by another person, this person must also be careful when removing the cartridge and needle and disposing of the cartridge and needle to prevent accidental needle stick injury and passing infection
How Do I Care for the Follistim Pen?
- Clean all exposed surfaces of the Follistim Pen with a clean, damp cloth such as a paper towel. Never wash it in water, detergent or strong medical cleaners.
- Handle the Follistim Pen carefully to avoid causing damage. You could damage the Follistim Pen by dropping it or handling it roughly.
- Keep the Follistim Pen away from dust and dirt.
- Never store the Follistim Pen with a needle attached to it. If you store the Follistim Pen with the needle attached, the drug could leak out and there is risk of contamination.
- If the Follistim Pen breaks or is damaged, do not try to fix it yourself. Contact your healthcare provider.
- Do not share your Follistim Pen with another person.
How should I store Follistim AQ Cartridge?
- Store Follistim AQ Cartridge in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date
- Follistim AQ can be stored at or below 77°F (25°C) for 3 months or until the expiration date, whichever comes first. Once the rubber inlay of the Follistim AQ Cartridge has been pierced by a needle, the product may be stored only for a maximum of 28 days at 36°F to 77°F (2°C to 25°C)
- Keep Follistim AQ Cartridge away from light
- Do not freeze
Keep Follistim AQ Cartridge, needles, and the disposal container, out of the reach of children.
Manufactured for Organon USA Inc.
Roseland, NJ 07068
by Vetter Pharma-Fertigung GmbH & Co. KG
Ravensburg, Germany
and packaged by Organon (Ireland) Ltd.
Swords, Co. Dublin, Ireland
BD, BD Logo and BD Micro-Fine are trademarks of Becton, Dickinson and Company
U.S. Patent Nos. 5,767,251; 5,929,028; 7,446,090 and 7,563,763.
Copyright © 2004, 2010 N.V. Organon. All rights reserved.
Rev. 9/10
34306400T
B-33554010
PRINCIPAL DISPLAY PANEL - 300 IU Carton
NDC 0052-0313-01
1 Sterile Prefilled 1.5 mL Cartridge
containing 0.420 mL and
5 BD Micro-Fine™ Pen Needles
Follistim®AQ Cartridge 300 IU
(follitropin beta injection)
For use only with Follistim Pen®,
available separately
For Subcutaneous Use
Organon
Rx only

PRINCIPAL DISPLAY PANEL - 600 IU Carton
NDC 0052-0316-01
1 Sterile Prefilled 1.5 mL Cartridge
containing 0.780 mL and
7 BD Micro-Fine™ Pen Needles
Follistim®AQ Cartridge 600 IU
(follitropin beta injection)
For use only with Follistim Pen®,
available separately
For Subcutaneous Use
Organon
Rx only

PRINCIPAL DISPLAY PANEL - 900 IU Carton
NDC 0052-0326-01
1 Sterile Prefilled 1.5 mL Cartridge
containing 1.170 mL and
10 BD Micro-Fine™ Pen Needles
Follistim®AQ Cartridge 900 IU
(follitropin beta injection)
For use only with Follistim Pen®,
available separately
For Subcutaneous Use
Organon
Rx only

| FOLLISTIM AQ
follitropin injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021211 | 06/28/2010 | |
| FOLLISTIM AQ
follitropin injection, solution |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021211 | 06/28/2010 | |
| FOLLISTIM AQ
follitropin injection, solution |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021211 | 06/28/2010 | |
| FOLLISTIM AQ
follitropin injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021211 | 06/28/2010 | |
| Labeler - Organon Pharmaceuticals USA (002152858) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vetter Pharma Fertigung GMBH and CO KG | 316126754 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vetter Pharma Fertigung GMBH and CO KG | 312670654 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| ORGANON IRELAND LTD | 219542271 | MANUFACTURE | |


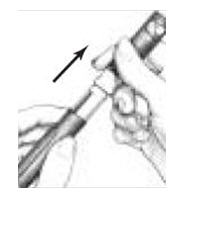
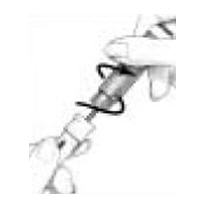
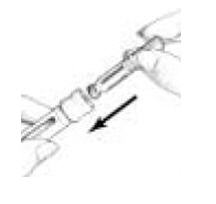
 on the Cartridge Holder should point to the middle of the yellow alignment mark
on the Cartridge Holder should point to the middle of the yellow alignment mark
 on the blue Pen Body
on the blue Pen Body