VIIBRYD
-
vilazodone hydrochloride tablet, film coated
VIIBRYD
-
vilazodone hydrochloride
Trovis Pharmaceuticals LLC
----------
|
||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of VIIBRYD or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. VIIBRYD is not approved for use in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4), and Patient Counseling Information (17.1)]
1 INDICATIONS AND USAGE
VIIBRYD is indicated for the treatment of major depressive disorder (MDD). The efficacy of VIIBRYD was established in two 8-week, randomized, double-blind, placebo-controlled trials in adult patients with a diagnosis of MDD [see Clinical Studies (14)].
Major depressive disorder consists of one or more major depressive episodes. A major depressive episode (DSM-IV-TR) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least 5 of the following 9 symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or a suicide attempt or suicidal ideation.
2 DOSAGE AND ADMINISTRATION
2.1 Initial Treatment of Major Depressive Disorder
The recommended dose for VIIBRYD is 40 mg once daily. VIIBRYD should be titrated, starting with an initial dose of 10 mg once daily for 7 days, followed by 20 mg once daily for an additional 7 days, and then an increase to 40 mg once daily. VIIBRYD should be taken with food. VIIBRYD blood concentrations (AUC) in the fasted state can be decreased by approximately 50% compared to the fed state, and may result in diminished effectiveness in some patients [see Pharmacokinetics (12.3)].
2.2 Maintenance/Continuation/Extended Treatment
The efficacy of VIIBRYD has not been systematically studied beyond 8 weeks. It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy. Patients should be reassessed periodically to determine the need for maintenance treatment and the appropriate dose for treatment.
2.3 Dosing in Special Populations
Pregnant Women: Neonates exposed to serotonergic antidepressants late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. When treating pregnant women with VIIBRYD, consider whether the potential benefits outweigh the potential risks of treatment [see Pregnancy (8.1)].
Nursing Mothers: There are no clinical data regarding the effect of VIIBRYD on lactation and nursing [see Nursing Mothers (8.3)]. Breastfeeding in women treated with VIIBRYD should be considered only if the potential benefit outweighs the potential risk.
Pediatric Patients: The safety and efficacy of VIIBRYD have not been studied in pediatric patients [see Pediatric Use (8.4)].
Geriatric Patients: No dose adjustment is recommended on the basis of age [see Geriatric Use (8.5)].
Hepatic Impairment: No dose adjustment is recommended in patients with mild or moderate hepatic impairment. VIIBRYD has not been studied in severe hepatic impairment [see Hepatic Impairment (8.6)].
Renal Impairment: No dose adjustment is recommended in patients with mild, moderate, or severe renal impairment. [see Renal Impairment (8.7)].
Gender: No dose adjustment is recommended on the basis of gender [see Gender Effect (8.8)].
2.4 Discontinuing Treatment
Discontinuation symptoms have been reported with discontinuation of serotonergic drugs such as VIIBRYD. Gradual dose reduction is recommended, instead of abrupt discontinuation, whenever possible. Monitor patients for these symptoms when discontinuing VIIBRYD. If intolerable symptoms occur following a dose decrease or upon discontinuation of treatment, consider resuming the previously prescribed dose and decreasing the dose at a more gradual rate [see Warnings and Precautions (5.6)].
2.5 Monoamine Oxidase Inhibitors (MAOI)
At least 14 days must elapse between discontinuation of an MAOI and initiation of therapy with VIIBRYD. In addition, at least 14 days must be allowed after stopping VIIBRYD before starting an MAOI [see Contraindications (4.1)].
3 DOSAGE FORMS AND STRENGTHS
VIIBRYD Tablets are available as 10 mg, 20 mg and 40 mg immediate-release, film-coated tablets.
10 mg pink, oval tablet, debossed with 10 on one side
20 mg orange, oval tablet, debossed with 20 on one side
40 mg blue, oval tablet, debossed with 40 on one side
4 CONTRAINDICATIONS
4.1 Monoamine Oxidase Inhibitors
VIIBRYD must not be used concomitantly in patients taking MAOIs or in patients who have taken MAOIs within the preceding 14 days due to the risk of serious, sometimes fatal, drug interactions with serotonergic drugs. These interactions have been associated with symptoms that include tremor, myoclonus, diaphoresis, nausea, vomiting, flushing, dizziness, hyperthermia with features resembling neuroleptic malignant syndrome, seizures, rigidity, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. Allow at least 14 days after stopping VIIBRYD before starting an MAOI [see Drug Interactions (7.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled studies of antidepressant drugs (selective serotonin reuptake inhibitors [SSRIs] and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with MDD and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled studies in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term studies of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled studies in adults with MDD or other psychiatric disorders included a total of 295 short-term studies (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18-24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25-64 | 1 fewer case |
| ≥65 | 6 fewer cases |
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms [see Warnings and Precautions (5.6) and Dosage and Administration (2.4)].
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for VIIBRYD should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose [see also Patient Counseling Information (17.1)].
Screening patients for bipolar disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled studies) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that VIIBRYD is not approved for use in treating bipolar depression.
5.2 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions has been reported with antidepressants alone, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs that impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Symptoms of serotonin syndrome were noted in 0.1% of patients treated with VIIBRYD. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble NMS, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of VIIBRYD with MAOIs intended to treat depression is contraindicated. [see Contraindications (4.1)].
If concomitant treatment of VIIBRYD with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Drug Interactions (7.1)].
The concomitant use of VIIBRYD with serotonin precursors (such as tryptophan) is not recommended [see Drug Interactions (7.1)].
Treatment with VIIBRYD and any concomitant serotonergic (SSRI, serotonin–norepinephrine reuptake inhibitor [SNRI], triptan, buspirone, tramadol, etc.) or antidopaminergic drugs, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
5.3 Seizures
VIIBRYD has not been systematically evaluated in patients with a seizure disorder. Patients with a history of seizures were excluded from clinical studies. Like other antidepressants, VIIBRYD should be prescribed with caution in patients with a seizure disorder.
5.4 Abnormal Bleeding
The use of drugs that interfere with serotonin reuptake inhibition, including VIIBRYD, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Patients should be cautioned about the risk of bleeding associated with the concomitant use of VIIBRYD and NSAIDs, aspirin, or other drugs that affect coagulation or bleeding.
5.5 Activation of Mania/Hypomania
Symptoms of mania/hypomania were reported in 0.1% of patients treated with VIIBRYD in clinical studies. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorder who were treated with other antidepressants. As with all antidepressants, use VIIBRYD cautiously in patients with a history or family history of bipolar disorder, mania, or hypomania.
5.6 Discontinuation of Treatment with VIIBRYD
There have been reports of adverse events occurring upon discontinuation of serotonergic antidepressants, particularly when discontinuation is abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
Monitor patients for these symptoms when discontinuing VIIBRYD. Reduce the dose gradually whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, consider resuming the previously prescribed dose. Subsequently, the dose may be decreased, but at a more gradual rate [see Dosage and Administration, (2.4)].
5.7 Hyponatremia
Although no cases of hyponatremia resulting from VIIBRYD treatment were reported in the clinical studies, hyponatremia has occurred as a result of treatment with SSRIs and SNRIs. In many cases, hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs. Also, patients taking diuretics or who are otherwise volume depleted can be at greater risk. Discontinuation of VIIBRYD in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
The most commonly observed adverse reactions in VIIBRYD-treated MDD patients in placebo-controlled studies (incidence ≥ 5% and at least twice the rate of placebo) were: diarrhea, nausea, vomiting, and insomnia.
Patient Exposure
The safety of VIIBRYD was evaluated in 2,177 patients (18-70 years of age) diagnosed with MDD who participated in clinical studies, representing 552 patient-years of exposure. In an open-label 52 week study at 40 mg daily, 599 patients were exposed to VIIBRYD for a total of 348 patient-years.
The information presented in these sections was derived from studies of VIIBRYD 40 mg daily in major depressive disorder including: 1) 2 placebo-controlled 8-week studies in 861 patients, including 436 receiving vilazodone; and 2) an open-label 52-week study of 599 patients. These studies included a titration period of 10 mg daily for 7 days followed by 20 mg daily for 7 days. In these clinical trials, VIIBRYD was administered with food.
Because clinical trials are conducted under widely varying conditions and varying lengths of time, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice.
Adverse reactions reported as reasons for discontinuation of treatment
In the placebo-controlled studies of MDD there was no single adverse reaction leading to discontinuation in > 1% of the patients. Overall, 7.1% of the patients who received VIIBRYD discontinued treatment due to an adverse reaction, compared with 3.2% of placebo-treated patients in these studies.
Common adverse reactions in placebo-controlled MDD studies
Table 2 shows the incidence of common adverse reactions that occurred in ≥ 2% of VIIBRYD-treated MDD patients (and greater than in placebo-treated patients) in the placebo-controlled studies.
| *Includes restlessness, akathisia, and restless legs syndrome | ||
| **Includes orgasm abnormal and anorgasmia | ||
| ***Male patients only (Placebo n=182; VIIBRYD n=170) | ||
| System Organ Class
Preferred Term | VIIBRYD
40 mg/day N = 436 | Placebo
N = 433 |
| Gastrointestinal disorders | ||
| Diarrhea | 28 | 9 |
| Nausea | 23 | 5 |
| Dry mouth | 8 | 5 |
| Vomiting | 5 | 1 |
| Dyspepsia | 3 | 2 |
| Flatulence | 3 | 2 |
| Gastroenteritis | 3 | <1 |
| Nervous system disorders | ||
| Dizziness | 9 | 5 |
| Somnolence | 3 | 2 |
| Paresthesia | 3 | 1 |
| Tremor | 2 | 0 |
| Psychiatric disorders | ||
| Insomnia | 6 | 2 |
| Abdnormal dreams | 4 | 1 |
| Libido decreased | 4 | <1 |
| Restlessness * | 3 | <1 |
| Orgasm abnormal ** | 3 | 0 |
| General disorders | ||
| Fatigue | 4 | 3 |
| Feeling jittery | 2 | <1 |
| Cardiac disorders | ||
| Palpitations | 2 | <1 |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 3 | 2 |
| Reproductive system and breast disorders | ||
| Delayed ejaculation*** | 2 | 0 |
| Erectile dysfunction*** | 2 | 1 |
| Metabolism and nutrition disorders | ||
| Increased appetite | 2 | 1 |
| − Not applicable | ||||
| *Includes anorgasmia | ||||
| Males | Females | |||
| Preferred Term | VIIBRYD
N= 170 | Placebo
N= 182 | VIIBRYD
N=266 | Placebo
N=251 |
| Decreased libido | 5 | 0 | 3 | <1 |
| Abnormal orgasm* | 4 | 0 | 2 | 0 |
| Delayed ejaculation | 2 | 0 | - | - |
| Erectile dysfunction | 2 | 1 | - | - |
| Sexual dysfunction | 2 | 0 | <1 | <1 |
Laboratory Tests
VIIBRYD has not been associated with any clinically important changes in laboratory test parameters in serum chemistry (including liver function tests), hematology and urinalysis, as measured in placebo-controlled studies. These studies include analysis of (1) mean change from baseline and (2) the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
ECG
VIIBRYD has not been associated with any clinically significant effect on ECG parameters, including QT, QTc, PR and QRS intervals, or with any arrhythmogenic potential. ECGs were evaluated in a thorough QTc study at doses up to 80 mg daily with food and in the placebo-controlled studies [see Pharmacodynamics (12.2)].
Vital Signs
VIIBRYD has not been associated with any clinically significant effect on vital signs, including systolic and diastolic blood pressure and heart rate, as measured in placebo-controlled studies. These studies included analyses of (1) change from baseline, and (2) the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
Weight
VIIBRYD had no effect on body weight as measured by the mean change from baseline in the 8-week, placebo-controlled studies. The mean changes in weight were +0.16 kg in the VIIBRYD group and +0.18 kg in the placebo group. The proportions of patients with a weight gain ≥ 7% were 0.9% in the VIIBRYD group and 1.2% in the placebo group. The proportions of patients with a weight decrease ≥ 7% were 1.4% in the VIIBRYD group and 1.4% in the placebo group.
Other adverse reactions observed in clinical studies
The following listing does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients:
Cardiac disorders: infrequent: ventricular extrasystoles
Eye disorders: frequent: vision blurred, dry eye; infrequent: cataracts
General disorders: infrequent: feeling abnormal
Metabolism and nutrition disorders: frequent: decreased appetite
Nervous System: frequent: sedation, migraine; infrequent: dysgeusia
Psychiatric disorders: infrequent: panic attack, mania
Renal and Urinary disorder: infrequent: pollakiuria
Skin and subcutaneous tissue disorders: frequent: hyperhidrosis, night sweats
7 DRUG INTERACTIONS
7.1 Central Nervous System (CNS)-Active Agents
The risk of using VIIBRYD in combination with other CNS-active drugs has not been systematically evaluated. Consequently, use caution when VIIBRYD is prescribed in combination with other CNS-active drugs.
Monoamine Oxidase Inhibitors (MAOI)
Adverse reactions, some of which are serious or fatal, can develop in patients who use MAOIs or who have recently been discontinued from a MAOI and started on antidepressant(s) with pharmacological properties similar to VIIBRYD (e.g. SSRIs), or who have recently had SSRI therapy discontinued prior to initiation of an MAOI. Do not prescribe VIIBRYD concomitantly with an MAOI or within 14 days of discontinuing or starting an MAOI [see Contraindications (4.1)].
Serotonergic Drugs
Based on the mechanism of action of VIIBRYD and the potential for serotonin toxicity, also known as serotonin syndrome, caution is advised when VIIBRYD is coadministered with other drugs that may affect the serotonergic neurotransmitter systems (e.g., MAOI, SSRIs, SNRIs, triptans, buspirone, tramadol, and tryptophan products etc.) [see Warnings and Precautions (5.2)].
7.2 Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. These studies have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs and SNRIs are coadministered with warfarin. Patients receiving warfarin therapy should be carefully monitored when VIIBRYD is initiated or discontinued [see Abnormal Bleeding (5.4)].
7.3 Potential for Other Drugs to Affect Vilazodone
Figure 1. Impact of other drugs on Vilazodone PK
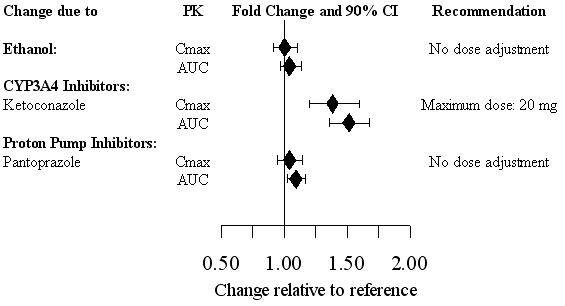
Inhibitors of CYP3A4
Metabolism by CYP3A4 is a major elimination pathway for vilazodone. Concomitant use of VIIBRYD and strong inhibitors of CYP3A4 (e.g., ketoconazole) can increase vilazodone plasma concentrations by approximately 50% (see Figure 1). The VIIBRYD dose should be reduced to 20 mg if co-administered with a strong inhibitor of CYP3A4. During co-administration with moderate inhibitors of CYP3A4 (e.g., erythromycin), the VIIBRYD dose should be reduced to 20 mg for patients with intolerable adverse events. No dose adjustment is recommended when VIIBRYD is co-administered with mild inhibitors of CYP3A4 (e.g., cimetidine).
Inducers of CYP3A4
Concomitant use of VIIBRYD with inducers of CYP3A4 has the potential to reduce vilazodone systemic exposure. However, the effect of CYP3A4 inducers on vilazodone plasma concentrations has not been evaluated.
Inhibitors of other CYP enzymes
Concomitant administration of VIIBRYD with inhibitors of CYP2C19 and CYP2D6 is not expected to alter plasma concentrations of vilazodone. These isoforms are minor elimination pathways in the metabolism of vilazodone. In vitro studies have shown that CYP1A2, CYP2A6, CYP2C9 and CYP2E1 have minimal contribution to the metabolism of vilazodone.
7.4 Potential for Vilazodone to Affect Other Drugs
Drugs metabolized by CYP1A2, CYP2C9, CYP2D6, CYP3A4 or CYP2C19.
Coadministration of VIIBRYD with substrates for CYP1A2, CYP2C9, CYP3A4, or CYP2D6 is unlikely to result in clinically significant changes in the concentrations of the CYP substrates. A study in healthy subjects found that VIIBRYD (20 mg/day for 8-10 days) had no effect on the pharmacokinetics of caffeine, flurbiprofen, nifedipine or debrisoquine, probes for CYP1A2, CYP2C9, CYP3A4, and CYP2D6, respectively. VIIBRYD coadministration with mephenytoin to healthy subjects resulted in a small (11%) increase in mephenytoin biotransformation, suggestive of a minor induction of CYP2C19. In vitro studies have shown that VIIBRYD is a moderate inhibitor of CYP2C19 and CYP2D6.
Drugs metabolized by CYP2C8
Coadministration of VIIBRYD with a CYP2C8 substrate may lead to an increase in concentration of the other drug. In vitro studies suggest that VIIBRYD may inhibit the biotransformation of substrates of CYP2C8. The effect of VIIBRYD on CYP2C8 activity has not been tested in vivo.
Induction of CYP isoforms
VIIBRYD did not induce CYP1A1, 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, 3A4 or 3A5 in an in vitro study in cultured human hepatocytes. Chronic administration of vilazodone is unlikely to induce the metabolism of drugs metabolized by these major CYP isoforms.
7.5 Drugs Highly Bound to Plasma Protein
The interaction between vilazodone and other highly protein-bound drugs has not been evaluated. Because vilazodone is highly bound to plasma protein, administration of VIIBRYD to a patient taking another drug that is highly protein bound may cause increased free concentrations of the other drug.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C
Vilazodone caused some developmental toxicity in rats, but was not teratogenic in rats or rabbits. There are no adequate and well-controlled studies of VIIBRYD in pregnant women. When treating pregnant women with VIIBRYD, carefully consider whether the potential benefits outweigh the potential risks of treatment.
No teratogenic effects were observed when vilazodone was given to pregnant rats or rabbits during the period of organogenesis at oral doses up to 200 and 36 mg/kg/day, respectively. These doses are 48 and 17 times, in rats and rabbits, respectively, the maximum recommended human dose (MRHD) of 40 mg on a mg/m2 basis. Fetal body weight gain was reduced, and skeletal ossification was delayed in both rats and rabbits at these doses; these effects were not observed at doses up to 10 times the MRHD in rats or 4 times the MRHD in rabbits.
When vilazodone was administered to pregnant rats at an oral dose of 30 times the MRHD during the period of organogenesis and throughout pregnancy and lactation, the number of live born pups was decreased. There was an increase in early postnatal pup mortality, and among surviving pups there was decreased body weight, delayed maturation, and decreased fertility in adulthood. There was some maternal toxicity at this dose. These effects were not seen at 6 times the MRHD.
Nonteratogenic Effects
Neonates exposed to serotonergic antidepressants late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of serotonergic antidepressants or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.2)].
8.2 Labor and Delivery
The effect of VIIBRYD on labor and delivery in humans is unknown. VIIBRYD should be used during labor and delivery only if the potential benefit outweighs the potential risk.
8.3 Nursing Mothers
Vilazodone is excreted into the milk of lactating rats. The effect of VIIBRYD on lactation and nursing in humans is unknown. Breast feeding in women treated with VIIBRYD should be considered only if the potential benefit outweighs the potential risk to the child.
8.4 Pediatric Use
Clinical studies on the use of VIIBRYD in pediatric patients have not been conducted; therefore, the safety and effectiveness of VIIBRYD in the pediatric population have not been established. VIIBRYD is not approved for use in pediatric patients [see Box Warning and Warnings and Precautions (5.1)].
8.5 Geriatric Use
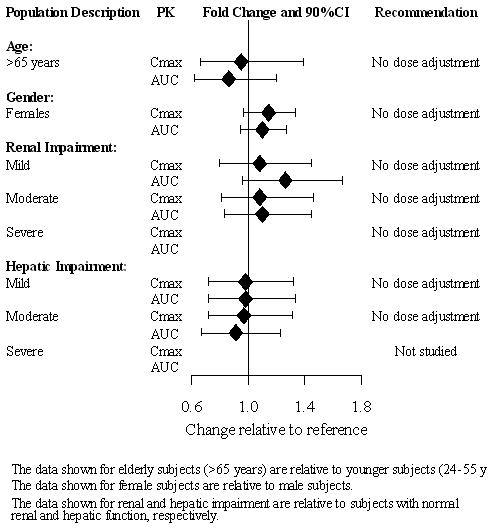
No dose adjustment is recommended on the basis of age (see Figure 2). Results from a single-dose (20 mg) pharmacokinetic study in elderly (> 65 years-old) vs. young (24-55 years-old) subjects demonstrated that the pharmacokinetics were generally similar between the two age groups.
Of the 2177 patients in clinical studies with VIIBRYD, 37 (1.7%) were 65 years of age or older, and 272 (12.5%) were 55 to 64 years of age.
Greater sensitivity of some older individuals cannot be ruled out [see Dosage and Administration (2.3)].
Serotonergic antidepressants have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event [see Warnings and Precautions (5.7)].
8.6 Hepatic Impairment
Vilazodone is eliminated primarily by hepatic metabolism. In mild and moderate hepatic impairment, no dose adjustment is necessary (see Figure 2). VIIBRYD has not been studied in patients with severe hepatic impairment [see Dosage and Administration (2.3)].
8.7 Renal Impairment
In mild, moderate, and severe renal impairment, no dose adjustment is necessary (see Figure 2 below) [see Dosage and Administration (2.3)].
8.8 Gender Effect
After adjustment for body weight, the systemic exposures between males and females are similar (see Figure 2).
Figure 2. Impact of Intrinsic Factors on Vilazodone PK
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
VIIBRYD is not a controlled substance.
9.2 Abuse and Dependence
VIIBRYD has been systematically studied in animals and did not demonstrate abuse or dependence potential. While VIIBRYD has not been systematically studied in humans for its potential for abuse, there was no suggested evidence of drug-seeking behavior in the clinical studies. However, it is not possible to predict on the basis of clinical experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of VIIBRYD (e.g., development of tolerance, drug-seeking behavior, increases in dose).
10 OVERDOSAGE
10.1 Human Experience
There is limited clinical experience regarding human overdosage with VIIBRYD. Four patients and 1 patient's child experienced an overdose of VIIBRYD; all recovered. The adverse reactions associated with overdose of VIIBRYD at doses of 200-280 mg as observed in clinical trials included serotonin syndrome, lethargy, restlessness, hallucinations, and disorientation.
10.2 Management of Overdose
Consult a Certified Poison Control Center for up-to-date guidance and advice. Telephone numbers for certified poison control centers are listed in the Physicians' Desk Reference® (PDR). No specific antidotes for vilazodone are known. In case of an overdose, provide supportive care, including close medical supervision and monitoring. Treatment should consist of those general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be considered. Removal of vilazodone by dialysis has not been studied; however, the high volume of distribution of vilazodone suggests that dialysis will not be effective in reducing vilazodone plasma concentrations.
11 DESCRIPTION
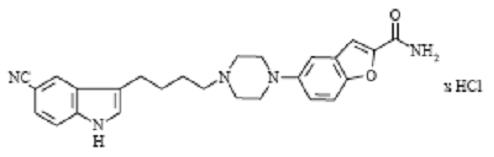
VIIBRYD Tablets for oral administration contain polymorph Form IV vilazodone hydrochloride (HCl), a selective serotonin reuptake inhibitor and a 5HT1A receptor partial agonist.
Vilazodone HCl is 2-benzofurancarboxamide, 5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-, hydrochloride (1:1). Its molecular weight is 477.99. The structural formula is:
In addition to the active ingredient, VIIBRYD Tablets contain lactose monohydrate, microcrystalline cellulose, magnesium stearate, colloidal silicon dioxide, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, FD&C Blue #1 (40 mg only), FD&C Yellow #6 (20 mg only) and FD&C Red #40 (10 mg only).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
The mechanism of the antidepressant effect of vilazodone is not fully understood but is thought to be related to its enhancement of serotonergic activity in the CNS through selective inhibition of serotonin reuptake. Vilazodone is also a partial agonist at serotonergic 5HT1A receptors; however , the net result of this action on serotonergic transmission and its role in vilazodone's antidepressant effect are unknown.
12.2 Pharmacodynamics
Vilazodone binds with high affinity to the serotonin reuptake site (Ki= 0.1 nM), but not to the norepinephrine (Ki=56 nM) or dopamine (Ki=37 nM) reuptake sites. Vilazodone potently and selectively inhibits reuptake of serotonin (IC50= 1.6 nM). Vilazodone also binds selectively with high affinity to 5-HT1A receptors (IC50=2.1 nM) and is a 5-HT1A receptor partial agonist.
Thorough QT Study: Treatment with VIIBRYD did not prolong the QTc interval. The effect of vilazodone (20, 40, 60, and 80 mg) on the QTc interval was evaluated in a randomized, placebo-, and active-controlled (moxifloxacin 400 mg), parallel-group, thorough QTc study in 157 healthy subjects. The study demonstrated an ability to detect small effects. The upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc interval was below 10 msec, based on the individual correction method (QTcI). This is below the threshold for clinical concern. However, it is unknown whether 80 mg is adequate to represent a high clinical exposure condition.
12.3 Pharmacokinetics
Vilazodone activity is due primarily to the parent drug. The pharmacokinetics of vilazodone (5 mg – 80 mg) are dose-proportional. Accumulation of vilazodone is predictable from single dose data, does not vary with dose, and steady-state is achieved in about 3 days. Elimination of vilazodone is primarily by hepatic metabolism with a terminal half-life of approximately 25 hours. At steady-state, after daily dosing of VIIBRYD 40 mg under fed conditions, the mean Cmax value is 156 ng/mL, and the mean AUC (0-24 hours) value is 1645 ng•h/mL.
Absorption
Vilazodone concentrations peak at a median of 4-5 hours (Tmax) after administration and decline with a terminal half-life of approximately 25 hours. The absolute bioavailability of vilazodone is 72% with food. Administration of VIIBRYD with food (high fat or light meal) increases oral bioavailability (Cmax increased by approximately 147-160%, and AUC increased by approximately 64-85%).
Coadministration of VIIBRYD with ethanol or with a proton pump inhibitor (pantoprazole) did not affect the rate or extent of vilazodone absorption [see Drug Interactions (7.3, Figure 1)]. In addition, neither the Tmax nor terminal elimination rate of vilazodone was altered by coadministration with either pantoprazole or ethanol.
Absorption is decreased by approximately 25% if vomiting occurs within 7 hours of ingestion; no replacement dose is needed.
Distribution
Vilazodone is widely distributed and approximately 96-99% protein-bound
Metabolism and Elimination
VIIBRYD is extensively metabolized through CYP and non-CYP pathways (possibly by carboxylesterase), with only 1% of the dose recovered in the urine and 2% of the dose recovered in the feces as unchanged vilazodone. CYP3A4 is primarily responsible for its metabolism among CYP pathways, with minor contributions from CYP2C19 and CYP2D6. In vitro studies with human microsomes and human hepatocytes indicate that vilazodone is unlikely to inhibit or induce the metabolism of other CYP (except for CYP2C8) substrates; and an in vivo study with probe substrates for CYP2C19, 2D6 and 3A4 showed vilazodone did not alter the pharmacokinetics of the probe substrates. However, an in vivo study with probe substrate for CYP2C19 demonstrated a minor induction of CYP2C19. Strong inhibitors of CYP3A4 (e.g., ketoconazole) can reduce the metabolism of vilazodone in vivo and increase exposure. Conversely, inducers of CYP3A4 can decrease vilazodone exposure [see Drug Interactions (7.3)].
The presence of mild or moderate renal impairment, or mild or moderate hepatic impairment did not affect the apparent clearance of vilazodone.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in which B6C3F1mice and Wistar rats were given oral doses of vilazodone up to 135 and 150 mg/kg/day, respectively, for 2 years. These doses are approximately 16.5 and 36 times the maximum recommended human dose (MRHD) of 40 mg, respectively, on a mg/m2 basis.
In mice, the incidence of hepatocellular carcinomas was increased in males at 16.5 times the MRHD; this finding was not observed at 5.5 times the MRHD. The incidence of malignant mammary gland tumors was numerically increased in females at 5.5 and 16.5 times the MRHD, with statistical significance at 16.5 the MHRD; this finding was not observed at 1.8 times the MRHD. Elevated prolactin levels were observed in a 2-week study of vilazodone administered at 5.5 and 33 times the MRHD. Increases in prolactin levels are known to cause mammary tumors in rodents.
In the rat study, vilazodone was not carcinogenic in either sex at doses up to 36 times the MRHD.
Mutagenesis
Vilazodone was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test). Vilazodone was negative in the in vitro V79/HGRPT mammalian cell forward mutation assay. Vilazodone was clastogenic in two in vitro mammalian cell chromosome aberration assays. However, vilazodone was negative for clastogenic activity in both an in vivo rat bone marrow chromosome aberration assay and a micronucleus test. Vilazodone was also negative in an in vivo/in vitro unscheduled DNA synthesis assay in rats.
Impairment of Fertility
Treatment of rats with vilazodone at a dose of 125 mg/kg, which is 30 times the maximum recommended human dose (MRHD) of 40 mg on a mg/m2 basis, caused impairment of male fertility with no effect on female fertility. Impaired male fertility was not observed at 6 times the MRHD.
14 CLINICAL STUDIES
The efficacy of VIIBRYD as a treatment for major depressive disorder was established in two 8-week, multicenter, randomized, double-blind, placebo-controlled studies in adult (18-70 years of age) outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD. In these studies, patients were titrated over 2 weeks to a dose of 40 mg of VIIBRYD with food (n=436) or placebo (n = 433) once daily. VIIBRYD was superior to placebo in the improvement of depressive symptoms as measured by the mean change from baseline to Week 8 in the Montgomery-Asberg Depression Rating Scale (MADRS) total score. Examination of population subgroups based on age (there were few patients over 65), gender, and race did not reveal any clear evidence of differential responsiveness.
| a Least Squares Mean (95% Confidence Interval) | ||
| Study Number | Primary Endpoint | LS Mean (95% CI) a difference from placebo in change from baseline |
| 1 | MADRS | -3.2 (-5.2, -1.3) |
| 2 | MADRS | -2.5 (-4.4, -0.6) |
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VIIBRYD (vilazodone HCl) Tablets are supplied in the following configurations:
10 mg, pink, oval tablet, debossed with 10 on one side
75838-110-30: 30-count bottles
75838-110-90: 90-count bottles
75838-110-52: 500-count bottles
75838-110-12: 10 blisters cards each containing 10 tablets (HUD)
20 mg, orange, oval tablet, debossed with 20 on one side
75838-120-30: 30-count bottles
75838-120-90: 90-count bottles
75838-120-52: 500-count bottles
75838-120-12: 10 blisters cards each containing 10 tablets (HUD)
40 mg, blue, oval tablet, debossed with 40 on one side
75838-140-30: 30-count bottles
75838-140-90: 90-count bottles
75838-140-52: 500-count bottles
75838-140-12: 10 blisters cards each containing 10 tablets (HUD)
Patient Starter Kit
75838-179-30: blister card containing 30 tablets:
10 mg, pink, oval, debossed with 10 on one side: 7 tablets
20 mg, orange, oval, debossed with 20 on one side: 7 tablets
40 mg, blue, oval, debossed with 40 on one side: 16 tablets
16.2 Storage
VIIBRYD (vilazodone HCl) Tablets should be stored at 25°C (77°F) with excursions permitted to 15°C - 30°C (59°F - 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See Medication Guide (17.2).
17.1 Information for Patients
Advise patients and their caregivers about the benefits and risks associated with treatment with VIIBRYD and counsel them in its appropriate use. Advise patients and their caregivers to read the Medication Guide and assist them in understanding its contents. The complete text of the Medication Guide is reprinted at the end of this document.
Suicide Risk
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dose is adjusted up or down [see Box Warning and Warnings and Precautions (5.1)].
Dosing and Administration
Instruct patients to take VIIBRYD with food. When initiating treatment with VIIBRYD the dose should be titrated, starting with a dose of 10 mg once daily for 7 days, followed by 20 mg once daily for an additional 7 days, and then increased to 40 mg once daily.
Concomitant Medication
Instruct patients not to take VIIBRYD with an MAOI or within 14 days of stopping an MAOI and to allow 14 days after stopping VIIBRYD before starting an MAOI [see Contraindications (4.1)].
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
Caution patients about the risk of serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions, particularly with the concomitant use of VIIBRYD and triptans, tramadol, tryptophan supplements, other serotonergic agents, or antipsychotic drugs [see Warnings and Precautions (5.2) and Drug Interactions (7.1)].
Seizures
Caution patients about using VIIBRYD if they have a history of a seizure disorder [see Warnings and Precautions (5.3)]. Patients with a history of seizures were excluded from clinical studies.
Abnormal Bleeding
Caution patients about the concomitant use of VIIBRYD and NSAIDs, aspirin, warfarin, or other drugs that affect coagulation since combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of abnormal bleeding [see Warnings and Precautions (5.4)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania [see Warnings and Precautions (5.5)].
Discontinuation
Advise patients not to stop taking VIIBRYD without talking first with their healthcare provider. Patients should be aware that discontinuation effects may occur when suddenly stopping VIIBRYD [see Warnings and Precautions (5.6)].
Hyponatremia
Advise patients that if they are treated with diuretics, or are otherwise volume depleted, or are elderly, they may be at greater risk of developing hyponatremia while taking VIIBRYD [see Warnings and Precautions (5.7)].
Alcohol
Advise patients to avoid alcohol while taking VIIBRYD [see Drug Interactions (7.3)].
Allergic Reactions
Advise patients to notify their healthcare provider if they develop an allergic reaction such as rash, hives, swelling, or difficulty breathing.
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy with VIIBRYD [see Use in Specific Populations (8.1)].
Nursing Mothers
Advise patients to notify their healthcare provider if they are breastfeeding an infant and would like to continue or start VIIBRYD [see Use in Specific Populations (8.3)].
Interference with Cognitive and Motor Performance
Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that VIIBRYD therapy does not adversely affect their ability to engage in such activities.
TROVIS PHARMACEUTICALS
Distributed by
Trovis Pharmaceuticals LLC
New Haven, CT 06511
877-878-7200
viibryd.com
Licensed from Merck KGaA,
Darmstadt, Germany
Product protected by U.S. Patent No. 5,532,241 and U.S. Patent No. 7,834,020.
VZ59PI0000
Revised: January 2011
VIIBRYD™ is a trademark of Trovis Pharmaceuticals LLC.
© 2011 Trovis Pharmaceuticals LLC.
17.2 Medication Guide
MEDICATION GUIDE
VIIBRYD [vī-brid]
(vilazodone hydrochloride)
Tablets
Read this Medication Guide carefully before you start taking VIIBRYD and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about VIIBRYD?
VIIBRYD and other antidepressant medicines may cause serious side effects.
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if there is an emergency:
-
Suicidal thoughts or actions:
- VIIBRYD and other antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
- Watch for these changes and call your healthcare provider right away if you notice:
- New or sudden changes in mood, behavior, actions, thoughts, or feelings, especially if severe.
- Pay particular attention to such changes when VIIBRYD is started or when the dose is changed.
Keep all follow-up visits with your healthcare provider and call between visits if you are worried about symptoms.
Call your healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive or violent
- thoughts about suicide or dying
- new or worse depression
- new or worse anxiety or panic attacks
- feeling agitated, restless, angry or irritable
- trouble sleeping
- an increase in activity or talking more than what is normal for you (mania)
- other unusual changes in behavior or mood
-
Serotonin Syndrome or Neuroleptic Malignant Syndrome-like reactions:
- agitation, hallucinations, coma or other changes in mental status
- coordination problems or muscle twitching (overactive reflexes)
- fast heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle stiffness or tightness
- Abnormal bleeding: VIIBRYD and other antidepressant medicines may increase your risk of bleeding or bruising, especially if you take the blood thinner warfarin (Coumadin®, Jantoven®), a non-steroidal anti-inflammatory drug (NSAID), or aspirin.
- Seizures or convulsions.
-
Manic episodes:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
-
Low salt (sodium) levels in the blood.
Elderly people may be at greater risk for this. Symptoms may include:- headache
- weakness or feeling unsteady
- confusion, problems concentrating or thinking or memory problems
Do not stop VIIBRYD without first talking to your healthcare provider. Stopping VIIBRYD suddenly may cause serious symptoms including:
- anxiety, irritability, high or low mood, feeling restless or sleepy
- headache, sweating, nausea, dizziness
- electric shock-like sensations, tremor, confusion
What is VIIBRYD?
VIIBRYD is a prescription medicine used to treat a certain type of depression called Major Depressive Disorder (MDD). It is important to talk with your healthcare provider about the risks of treating depression and also the risk of not treating it. You should discuss all treatment choices with your healthcare provider.
Talk to your healthcare provider if you do not think that your condition is getting better with VIIBRYD treatment.
It is not known if VIIBRYD is safe and effective in children.
Who should not take VIIBRYD?
Do not take VIIBRYD if you:
- Take an Monoamine Oxidase Inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI.
- Do not take an MAOI within 14 days of stopping VIIBRYD.
- Do not start VIIBRYD if you stopped taking an MAOI in the last 14 days.
People who take VIIBRYD close in time to taking an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:- high fever
- uncontrolled muscle spasms
- stiff muscles
- rapid changes in heart rate or blood pressure
- confusion
- loss of consciousness (pass out)
What should I tell my healthcare provider before taking VIIBRYD?
Before starting VIIBRYD, tell your healthcare provider if you:
- have liver problems
- have kidney problems
- have or had seizures or convulsions
- have bipolar disorder (manic depression) or mania
- have low sodium levels in your blood
- have or had bleeding problems
- drink alcohol
- have any other medical conditions
- Are pregnant or plan to become pregnant. It is not known if VIIBRYD will harm your unborn baby. Talk to your healthcare provider about the benefits and risks of treating depression during pregnancy.
- Are breastfeeding or plan to breastfeed. It is not known if VIIBRYD passes into breast milk. You and your healthcare provider should decide if you should take VIIBRYD while breastfeeding.
Tell your healthcare provider about all the medicines that you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. VIIBRYD and some medicines may interact with each other, may not work as well, or may cause serious side effects when taken together.
Especially tell your healthcare provider if you take:
- triptans used to treat migraine headache
- medicines used to treat mood, anxiety, psychotic or thought disorders, including tricyclics, lithium, SSRIs, SNRIs, buspirone, or antipsychotics
- tramadol
- over-the-counter supplements such as tryptophan or St. John’s Wort
- nonsteroidal anti-inflammatory drugs (NSAIDS)
- aspirin
- warfarin (Coumadin, Jantoven)
- mephenytoin (Mesantoin)
- diuretics
Your healthcare provider or pharmacist can tell you if it is safe to take VIIBRYD with your other medicines. Do not start or stop any medicine while taking VIIBRYD without talking to your healthcare provider first.
How should I take VIIBRYD?
- Take VIIBRYD exactly as prescribed. Your healthcare provider may need to change the dose of VIIBRYD until it is the right dose for you.
- Take VIIBRYD with food. VIIBRYD may not work as well if you take it on an empty stomach.
- If you miss a dose of VIIBRYD, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of VIIBRYD at the same.
- If you take too much VIIBRYD, call your healthcare provider or poison control center right away, or get emergency treatment.
What should I avoid while taking VIIBRYD?
- VIIBRYD can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how VIIBRYD affects you.
- You should avoid drinking alcohol while taking VIIBRYD. See "What should I tell my healthcare provider before taking VIIBRYD?"
What are the possible side effects of VIIBRYD?
VIIBRYD may cause serious side effects, including:
Common side effects in people who take VIIBRYD include:
- diarrhea
- nausea or vomiting
- trouble sleeping
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of VIIBRYD. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VIIBRYD?
Store VIIBRYD at room temperature (59°F to 86°F or 15°C to 30°C).
Keep VIIBRYD and all medicines out of the reach of children.
General information about VIIBRYD.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VIIBRYD for a condition for which it was not prescribed. Do not give VIIBRYD to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about VIIBRYD. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about VIIBRYD that is written for healthcare professionals.
For more information about VIIBRYD call 1-877-878-7200 or go to www.VIIBRYD.com.
What are the ingredients in VIIBRYD?
Active ingredient: vilazodone hydrochloride
Inactive ingredients: lactose monohydrate, microcrystalline cellulose, magnesium stearate, colloidal silicon dioxide, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and FD&C Blue #1 (40 mg only), FD&C Yellow #6 (20 mg only) and FD&C Red #40 (10 mg only).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Trovis Pharmaceuticals
Trovis Pharmaceuticals LLC
5 Science Park
New Haven, CT 06511
Licensed from Merck KGaA, Darmstadt, Germany
Product protected by U.S. Patent No. 5,532,241 and U.S. Patent No. 7,834,020.
VIIBRYD™ is a trademark of Trovis Pharmaceuticals LLC.
© 2011 Trovis Pharmaceuticals LLC.
Revised January2011
Package Label – Principle Display Panel – 10 mg Tablets 30 ct Bottle

Package Label – Principle Display Panel – 20 mg Tablets 30 ct Bottle

Package Label – Principle Display Panel – 40 mg Tablets 30 ct Bottle

Package Label – Principle Display Panel – Physician Sample Pack

Package Label – Principle Display Panel – Starter Kit
| VIIBRYD
vilazodone hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022567 | 04/18/2011 | |
| VIIBRYD
vilazodone hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022567 | 04/18/2011 | |
| VIIBRYD
vilazodone hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022567 | 04/18/2011 | |
| VIIBRYD
vilazodone hydrochloride kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022567 | 04/18/2011 | |
| VIIBRYD
vilazodone hydrochloride kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022567 | 04/18/2011 | |
| Labeler - Trovis Pharmaceuticals LLC (804037526) |