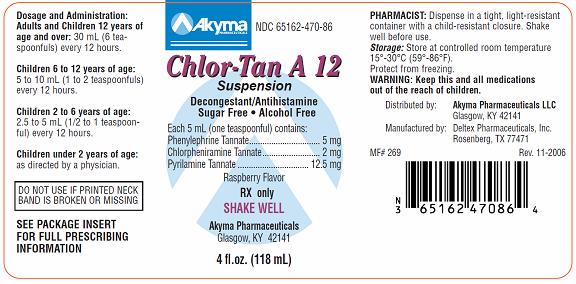
CHLOR-TAN A 12 SUSPENSION
-
phenylephrine tannate,
chlorpheniramine tannate and
pyrilamine tannate suspension
Amneal Pharmaceuticals
----------
Chlor-Tan A 12DESCRIPTION
Each 5 ml (one teaspoon) contains:
Phenylephrine Tannate . . . . . . . . . . 5 mg
Chlorpheniramine Tannate . . . . . . . . . . 2 mg
Pyrilamine Tannate . . . . . . . . 12.5 mg
Phenylephrine is a sympathomimetic amine used as a vasoconstrictor having the chemical name (-)-m- Hydroxy-α-[(methyl-amino)methyl]benzyl alcohol; C9H13N02, MW = 167.22. The tannate salt of phenylephrine has the empirical formula C76H52NO48 and MW = 1868.11. Chlorpheniramine is an antihistamine having the chemical name 2-[p-Chloro- α -[2-(dimethyl-amino)ethyl]benzyl]pyridine; C16H19ClN2, MW = 274.79.
The tannate salt of chlorpheniramine has the empirical formula C92H71ClN2O46 and MW = 1975.68. Pyrilamine is an antihistamine having the chemical name 2-[[(2-(Dimethyl-amino)ethyl](p-methoxybenzyl)amino]pyridine; C17H23N3O, MW = 285.39. The tannate salt of pyrilamine has the empirical formula C93H75N3047 and MW = 1986.28.
Chlor-Tan A 12 is a decongestant antihistamine suspension, which is red/pink in color with a raspberry flavor and odor.
Other ingredients: citric acid, FD&C Red #40, D&C Red #33, raspberry flavor, glycerin, methylparaben, sodium benzoate, sodium saccharin, xanthan gum, and purified water.
CLINICAL PHARMACOLOGY
Chlor-Tan A 12 Suspension combines the sympathomimetic decongestant effect of phenylephrine with the antihistaminic actions of chlorpheniramine and pyrilamine.
INDICATIONS AND USAGE
Chlor-Tan A 12 Suspension is indicated for sympathomimetic relief of the coryza and nasal congestion associated with allergic rhinitis, sinusitus, and other respiratory tract conditions. Appropriate therapy should be provided for the primary disease.
CONTRAINDICATIONS
Chlor-Tan A 12 Suspension is contraindicated for newborns, nursing mothers, and patients sensitive to any of the ingredients or related compounds.
WARNINGS
Use with caution in patients with hypertension, cardiovascular disease, hyperthyroidism, diabetes, narrow angle glaucoma or prostatic hypertrophy. Use with caution or avoid use in patients taking monoamine oxidase (MAO) inhibitors. This product contains antihistamines which may cause drowsiness and may have additive central nervous system (CNS) effects with alcohol or other CNS depressants (e.g., hypnotics, sedatives, tranquilizers).
PRECAUTIONS
General: Antihistamines are more likely to cause dizziness, sedation and hypotension in elderly patients. Antihistamines may cause excitation, particularly in children, but their combination with sympathomimetics may cause either mild stimulation or mild sedation.
Information for Patients: Caution patients against drinking alcoholic beverages or engaging in potentially hazardous activities requiring alertness, such as driving a car or operating machinery, while using this product.
Drug Interaction: Do not prescribe this product for use in patients that are now taking a prescription MAO inhibitor (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 14 days after stopping the MAO inhibitor drug therapy. MAO Inhibitors and betaadrenergic blockers increase the effects of sympathomimetics. Sympathomimetics may reduce the antihypertensive effects of methyldopa, mecamylamine, reserpine, and veratrum alkaloids. Phenylephrine tannate may result in additive CNS depressant effects when coadministered with alcohol, antihistamines, psychotropics, or other drugs which produce CNS depression.
Phenylephrine tannate should not be used with ganglionic blocking drugs, such as
guanethidine sulfate or bethanidine, since it antagonizes the hypotensive action of these drugs. Concurrent use of phenylephrine tannate with other sympathomimetic amines may increase pressure or cardiovascular effects of either medication.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term animal studies have been performed with Chlor-Tan A 12 Suspension.
Pregnancy: Teratogenic Effects — Pregnancy Category C: Animal reproduction studies have not been conducted with Chlor-Tan A 12 Suspension. It is also not known whether Chlor-Tan A 12 Suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Chlor-Tan A 12 Suspension should be given to a pregnant woman only if clearly needed.
Nursing Mothers: Chlor-Tan A 12 Suspension should not be administered to a nursing woman.
Pediatric Use: Safety and effectiveness of Chlor-Tan A 12 Suspension in pediatric patients have not been established by adequate and well-controlled clinical studies.
Geriatric Use: Chlor-Tan A 12 Suspension is known to be-substantially excreted by the kidneys, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
ADVERSE REACTIONS
Adverse effects associated with Chlor-Tan A 12 Suspension at recommended doses have been minimal. The most common have been drowsiness, sedation, dryness of mucous membranes and gastrointestinal effects. Serious side effects with oral antihistamines or
sympathomimetics have been rare. Sympathomimetic amines may cause tachycardia, palpitations, nervousness, excitability, dizziness, insomnia, restlessness, headache, gastric Irritation, and irritability. Sympathomimetic amines have been associated with certain untoward reactions including fear, anxiety, tenseness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, Insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension. Hyperactive
Individuals may display ephedrine-like reactions such as tachycardia, palpitations, headache, dizziness, or nausea. Urinary retention may occur in patients with prostatic hypertrophy.
OVERDOSAGE
Signs and symptoms may vary from ONS depression to stimulation restlessness to convulsions. Antihistamine overdosage in young children may lead to convulsions and death. Atropine-like signs and symptoms may be prominent.
Treatment: Induce vomiting if it has not occurred spontaneously. Precautions must be taken against aspiration, especially in infants, children and comatose patients. If gastric lavage is Indicated, isotonic or half-isotonic saline solution is preferred. Stimulants should not be used, if hypotension is a problem, vasopressor agents may be considered.
DOSAGE AND ADMINISTRATION
Chlor-Tan A 12 Suspension is administered orally as follows:
Adults and children 12 years of age and over: 30 mL (6 teaspoonfuls) every 12 hours. Children 6-12 years of age: 5 to 10 mL (1-2 teaspoonfuls) every 12 hours.
Children 2-6 years of age: 2.5 to 5 mL (1/2-1 teaspoonful) every 12 hours.
Children under 2 years of age: as directed by a physician.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSAGE, CONTACT A POISON CONTROL CENTER AND SEEK PROFESSIONAL ASSISTANCE IMMEDIATELY.
RECOMMENDED STORAGE:
Store at controlled room temperature, 15°C - 30°C (59°F - 86°F). Protect from freezing.
DISPENSE IN A TIGHT LIGHT-RESISTANT CONTAINER WITH CHILD-RESISTANT CLOSURES AS DEFINED IN THE USP/NF.
SHAKE WELL BEFORE USING.
HOW SUPPLIED
Chlor-Tan A 12 Suspension is supplied in bottles of 4 fl. oz. (118 mL)
NDC 65162-470-86.
Distributed by:
Akyma Pharmaceuticals LLC
Glasgow, KY 42141
Manufactured by:
Deltex Pharmaceuticals, Inc.
Rosenberg, TX 77471
MF# 268 Rev. 11-2006
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
| CHLOR-TAN A 12 SUSPENSION
phenylephrine tannate chlorpheniramine tannate and pyrilamine tannate suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 01/06/2006 | 12/05/2008 | |
| Labeler - Amneal Pharmaceuticals (123797875) |
| Registrant - Amneal Pharmaceuticals (123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Amneal Pharmaceuticals | 963900878 | ANALYSIS, LABEL, MANUFACTURE, PACK | |