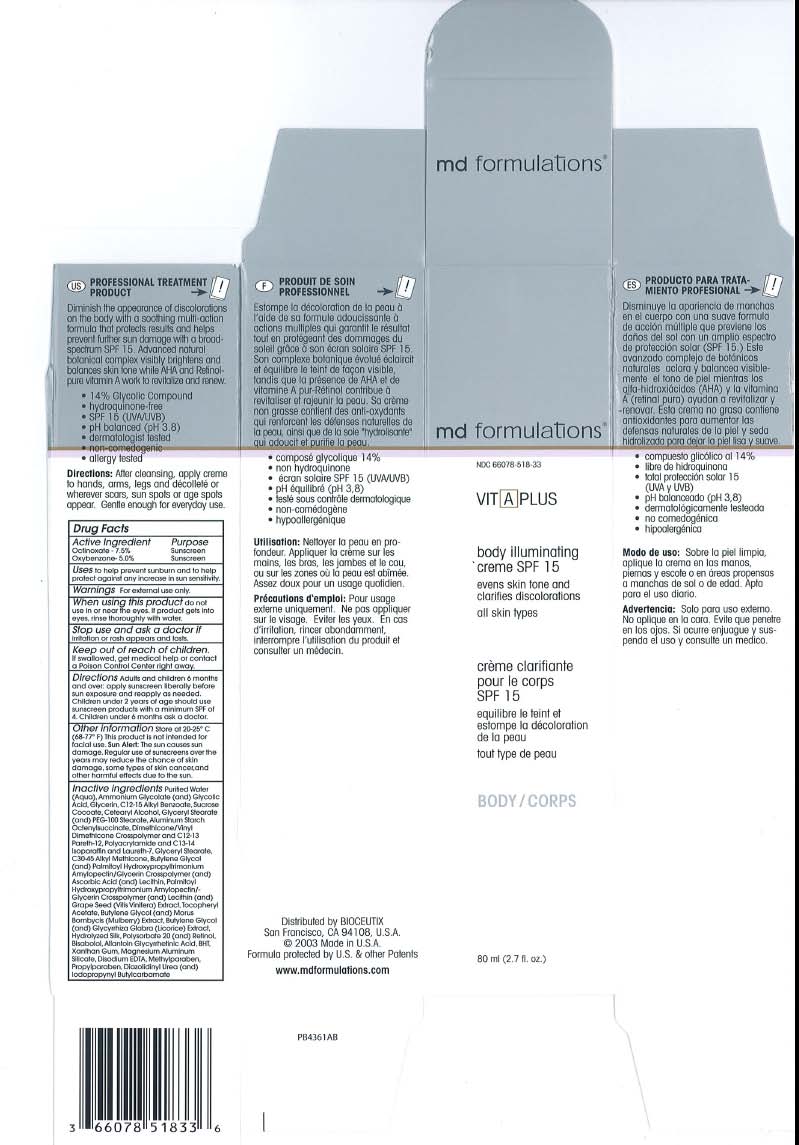
BODY ILLUMINATING CREME SPF 15
-
octinoxate and
oxybenzone cream
MD Formulation
----------
VitAplus body illuminating creme SPF 15Active Ingredient Purpose
Octinoxote - 7.5% Sunscreen
Oxybenzone- 5.0% Sunscreen
When using this product do not use in or near the eyes. II product gets into eyes, rinse thoroughly with water.
Warnings For external use only.
Porelh-12, Polyacrylamide and CI 3-14 lsoporaffin and laureth-7, Glyceryl Stearale. C30-45 Alkyl Methlcone, Butylene Glycol (and) Palmltoyl Hydroxypropyltrlmonlum Amylopectin/Glycerin Crosspolymer (and) Ascorbic Acid (and) l ecithin, Polmlloyl HydroxypropyHrimonlum Amylopectin/-
Glycerin Crosspolymer (and) lecithin (and) Grope Seed (Vrtis Vinilera) Exlracl, Tocopheryt Acetate, Butylene Glycol (and) Morus Bombycls (Mulberry) Extract, Butylene Glycol (and) Glycyrrhiza Glabra (Ucorlce) Exlract, Hydrolyzed Silk. Polysorbale 20 (and) Retinol. Blsobolol, Allantoin Glycyrrh ellnlc Acid, BHT. Xanthon Gum. Magnesium Aluminum Sillcale, Dlsodlum EDTA. Methylparoben. Propylparaben, Dlazolldinyl Urea (and)
lodoplopynyl Butylcorbamole Active Ingredient Purpose
Octinoxote - 7.5% Sunscreen
Oxybenzone- 5.0% Sunscreen Directions Adults and children 6 months and over: opply sunscreen liberally before sun exposure ond reapply as needed. Children under 2 years of age should use sunscreen products with a minimum SPF of 4. Children under 6 monlhs ask a doctor.

| BODY ILLUMINATING CREME SPF 15
octinoxate, oxybenzone cream |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part352 | 07/14/2003 | 04/06/2011 |
| Labeler - MD Formulation (087008363) |
| Registrant - Harmony Labs, Inc. (105803274) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Harmony Labs, Inc. | 105803274 | manufacture, label, pack, relabel, repack | |