LIDOWORX
-
lidocaine gel
DermWorx Incorporated
----------
LidoWorx™ Gel(4% Lidocaine)
Active Ingredient:
Lidocaine (4%)
Purpose:
Topical anesthetic
Use:
For the temporary relief of pain and itching on normal intact skin.
Warnings:
For external use only.
Flammable:
Keep away from open fire or flame.
When using this product
- avoid contact with eyes.
- do not use in large quantities, particularly over raw surfaces or blistered areas
Stop use and ask a doctor
if the condition worsens or if the symptoms clear up and occur again in a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions:
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: consult a doctor.
Other Information:
Store at controlled room temperature15-30 °C.
Inactive ingredients:
hexamethyldisiloxane, hydroxypropylcellulose, isopropyl alcohol, oleyl alcohol, NF, propylene glycol, USP, vanilla fragrance.
Patient Information
LIDOWORX™ Gel
(4% lidocaine)
Topical Anesthetic for Local or Dermal Analgesia
Description
LidoWorx™ incorporates DermWorx patented Small Molecule Solubilization System™ (SMSS™) for a very rapid and optimum onset of anesthetic action.
LidoWorx™ is an alcoholic, non-oily, translucent gel containing 4% lidocaine with skin permeation enhancers, indicated as a topical analgesic for use on normal intact skin for rapid local analgesia.
Composition
Each gram of LidoWorx™ contains lidocaine (40 mg) in a gel composed of hexamethyldisiloxane, hydroxypropylcellulose, isopropyl alcohol, oleyl alcohol NF, propylene glycol USP, and vanilla fragrance.
The chemical designation of lidocaine is 2-(diethylamino)-N(2,6-dimethylphenyl),acetamide; melting point 68-69°C; molecular weight 234.33. It has the following structure:
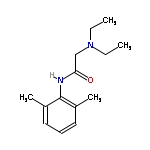
Mechanism of Action
LidoWorx™ applied to intact skin provides dermal analgesia by the release of lidocaine from the gel into the epidermis and dermis. Lidocaine is a local anesthetic agent of the amide type. Local anesthetics reversibly block the initiation and conduction of nerve impulses by interfering with the flux of sodium ions though the neuronal membrane. The onset, depth and duration of dermal analgesia provided by LidoWorx™ depend primarily on the site of application and duration of application. Dermal analgesia is obtained within 30 minutes of application, and the effect persists for approximately 30 minutes after removal. Areas such as the upper lip may become numb after 15 minutes of application. Dermal application of LidoWorx™ may cause local blanching followed by local redness or erythema. These effects are mild and transitory.
Pharmacokinetics
LidoWorx™ is formulated to insure local penetration of lidocaine into the skin. A side effect of this desired local effect may be the systemic absorption of lidocaine. The systemic absorption of lidocaine from LidoWorx™ has not been determined in clinical studies, but information available in the literature indicates that the amount of lidocaine systemically absorbed is directly related to both the duration of application and to the area over which it is applied. By inference from published clinical studies, the application of LidoWorx™ to broken or inflamed skin, or to 2,000 cm2 or more of skin of an adult, (600 cm2 in children 10-20 kg body weight, 100 cm2 in children up to 10 kg) could result in plasma levels that could, in susceptible individuals, produce a systemic pharmacologic response. Patients receiving lidocaine by prolonged infusion (for suppression of ventricular dysrhythmias) present objective systemic toxic effects at plasma concentrations of 6-10 µg/ml. When lidocaine is administered intravenously, it distributes to highly perfused organs such as brain, liver, kidney and heart. Lidocaine binds to plasma proteins (approx. 70%). It can cross the placental and blood brain barrier, presumably by passive diffusion. It is not known whether lidocaine is metabolized in the skin. When administered intravenously it is metabolized by the liver. The major metabolites are monoethylglycylxylidide (MEGX) and glycylxylidide (GX). The biological half-life of lidocaine is 1.5 hrs; its plasma clearance averages 1 L/min and is dependent on liver blood flow. Both MEGX and GX have pharmacologic effects, both as antiarrythmics and in terms of toxicity.
Indications and Usage
LidoWorx™ (4% lidocaine) is indicated for the temporary relief of pain and itching. It is a topical analgesic for penetrating local pain relief on normal intact skin.
LidoWorx™ is not recommended for use on mucous membranes because of much greater absorption than through intact skin. LidoWorx™ is not recommended for use beyond the external ear. Not for ophthalmic use.
Contraindication
LidoWorx™ is contraindicated in patients with sensitivity to the amide type local anesthetics or to any other component of the product.
Warning
For external use only.
Flammable: Keep away from open fire or flame.
Caution - Do not use in the eyes. Not for prolonged use. If the condition for which this preparation is used persists or if a rash develops, discontinue use and consult physician. Avoid contact with the eyes. Do not use over large areas of the body. Do not use for more than 7 days unless directed by a doctor. Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Inappropriate use of this product, such as application on mucous membranes, on large areas of the body or for long periods of time, or on individuals that are allergic to the amide type anesthetics, may result in serious side effects. Consultation with a doctor before using this product is strongly recommended.
Precautions
General
Repeated doses of LidoWorx™ may increase blood levels of lidocaine. Avoid contact with the eye since it may cause severe irritation and loss of protective reflexes. If eye contact occurs, immediately wash out the eye with water or saline. Patients with severe hepatic disease are at a greater risk of developing toxic plasma concentrations of lidocaine.
Information for Patients
When LidoWorx™ is used the patient should be aware that the production of dermal analgesia may be accompanied by the block of all sensations in the treated skin. For this reason, the patient should avoid inadvertent trauma to the treated area by scratching, rubbing or exposure to extreme hot or cold temperatures until complete sensation has returned.
Drug Interactions
LidoWorx™ should be used with caution in patients receiving Class I antiarrythmic agents (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Metabolites of lidocaine have been shown to be carcinogenic in laboratory animals. A two-year oral toxicity study of 2,6-xylidine, a metabolite of lidocaine, has shown that in both male and female rats daily doses of 900 mg/m2 (60 times SDA) resulted in adenomas and carcinomas of the nasal cavity. With daily doses of 300 mg/m2 (20 times SDA) the increase in incidence of nasal carcinomas and or adenomas in each sex of the rat were not statistically greater than the control group. In the low dose (90 mg/m2, 6 times SDA) and control groups, no nasal tumors were observed.
Mutagenesis
The mutagenic potential of lidocaine HCl has been tested in the Ames Salmonella -mammalian microsome test and by analysis of structural chromosome aberrations in human lymphocites in vitro, and by the mouse micronuclear test in vivo. There was no indication in these three tests of any mutagenic effects. The mutagenicity of 2,6-xylidine, a metabolite of lidocaine has been studied in different tests with mixed results. The compound was found to be weakly mutagenic in the Ames test only under metabolic activation conditions. In addition, 2,6-xylidine was observed to be mutagenic at the thymidine kinase locus, with or without activation, and induced chromosome aberrations and sister chromatid exchanges at concentrations in which the drug precipitated out of solution (1.2 mg/ml). No evidence of genotoxicity was found in the in vivo assays measuring unscheduled DNA synthesis in rat hepatocytes, chromosome damage in polychromatic erythrocytes or preferential killing of DNA repair-deficient bacteria in liver, lung kidney, testes and blood extracts from mice. However covalent binding studies of DNA from liver and ethmoid turbinates in rats indicate that 2,6 xylidine may be genotoxic under certain conditions in vivo.
Use in Pregnancy
Teratogenic Effects: Pregnancy Category B
Reproduction studies with lidocaine have been performed in rats and have revealed no evidence of harm to the fetus (30 mg/kg subcutaneously, 22 times SDA). There are however, no adequate and well-controlled studies on pregnant women. Therefore LidoWorx™ should be used in pregnancy only under a doctor's supervision.
Labor and Delivery
Lidocaine is not contraindicated in labor and delivery. Should LidoWorx™ be used concomitantly with other products containing lidocaine, total doses contributed by all formulations must be considered.
Nursing Mothers
Lidocaine is excreted in human milk, therefore, caution should be exercised when LidoWorx™ is administered to a nursing mother since the milk:plasma ratio of lidocaine is 0.4.
Pediatric Use
Consult a doctor. Observe child during application to avoid accidental ingestion. Not for otic or ophtalmic use.
Adverse Reactions
Localized Reactions
During or immediately after treatment with LidoWorx™ the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation. These effects are mild and transient, resolving spontaneously within 1 or 2 hours. Other common effects is paleness (pallor or blanching) when the application time is very prolonged (over 2 hours), and less frequently alterations in temperature sensations, edema, itching and rash.
Allergic Reactions
Allergic and anaphylactoid reactions associated with lidocaine can occur. They are characterized by urticaria, angioedema, bronchospasm, and shock.
Systemic (Dose Related) Reactions
Systemic adverse reactions following appropriate use of LidoWorx™ are unlikely. Systemic adverse effects of lidocaine are similar in nature to those observed with other amide type local anesthetics including CNS excitation and/or depression (light-headedness; nervousness; apprehension; euphoria; confusion; dizziness; drowsiness; tinnitus; blurred or double vision; vomiting; sensations of heat, cold or numbness; twitching; tremors; convulsions; unconsciousness; respiratory depression and arrest). Cardiovascular manifestations may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
Important
Not for ophthalmic use
Keep container closed at all times when not in use. Keep out of reach of children.
Patch test recommended: As with any topical, it is recommended that a patch test be performed to rule out any untoward effects. Apply on a small, inconspicuous area and observe for 24 hours. Preferably repeat this patch test 7 days after the initial one.
Directions for Use
Adults and children 2 years and older: Apply externally to the affected area not more than 3 to 4 times daily. Children under 2 years of age, consult a doctor.
Apply a moderately thick layer to the affected area (approximately 1/8 inch thick). Allow time, for numbness to develop, approximately 15 minutes. Best results are obtained 15 to 30 minutes post-application. Wipe-off excess gel. LidoWorx™ may be applied under an occlusive dressing.
How Supplied
LidoWorx™ 4% is supplied in the USA as a single use 0.4 oz (12 g) tube. Keep out of reach of children. This package is not child resistant, discard after initial use.
Please note that the tubes are filled by weight, not volume. Air bubbles may be present in the gel. Store at controlled room temperature, 15-30 ˚C
NDC # 28109-030-01
Distributed by:
DermWorx, Inc.
Hallandale, FL 33009
www.dermworx.com
1-877-dermworx
Principal Display Panel - Tube Label
Our patented Small Molecule Solubilization System™ provides a very rapid and optimum onset of anesthetic action.
Discard after Use.
For External Use Only.
Not for Ophthalmic Use.
Keep Out of Reach of Children.
DermWorx Incorporated
Hallandale, FL 33009
www.dermworx.com
1-877-dermworx
NDC 28109-030-01
LidoWorx™
Gel (4% lidocaine)
FOR PROFESSIONAL USE ONLY
Topical Anesthetic for local or dermal analgesia
0.4 oz (12 gm)

Tube Label
Principal Display Panel - Outer Carton Label
NDC 28109-030-06
LidoWorx™
Gel (4% lidocaine)
Patented Small Molecule Solubilization System™ (SMSS™) provides a very rapid and optimum onset of anesthetic action.
FOR PROFESSIONAL USE ONLY
Topical Anesthetic for local or dermal analgesia
DermWorx Incorporated
Hallandale, FL 33009
www.dermworx.com
1-877-dermworx
Outer Carton Label
| LIDOWORX
lidocaine gel |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part348 | 04/18/2009 | 04/06/2011 |
| Labeler - DermWorx Incorporated (015678067) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Harmony Labs | 105803274 | MANUFACTURE | |