COLD RELIEF
-
acetaminophen,
dextromethorphan hydrobromide,
guaifenesin and
phenylephrine hydrochloride tablet, coated
Meijer Distribution Inc
----------
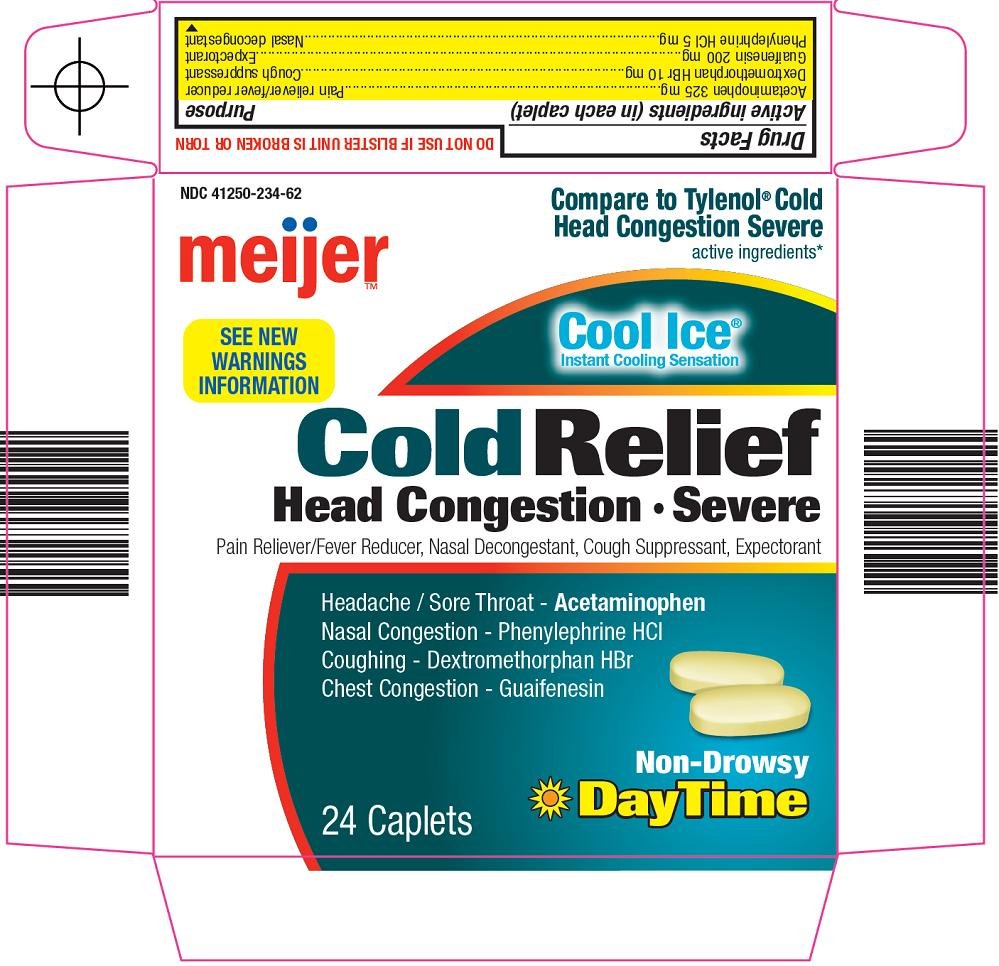
Meijer Distribution, Inc. Cold Relief Drug FactsActive ingredient (in each caplet)
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Guaifenesin 200 mg
Phenylephrine HCl 5 mg
Purpose
Pain reliever/fever reducer
Cough suppressant
Expectorant
Nasal decongestant
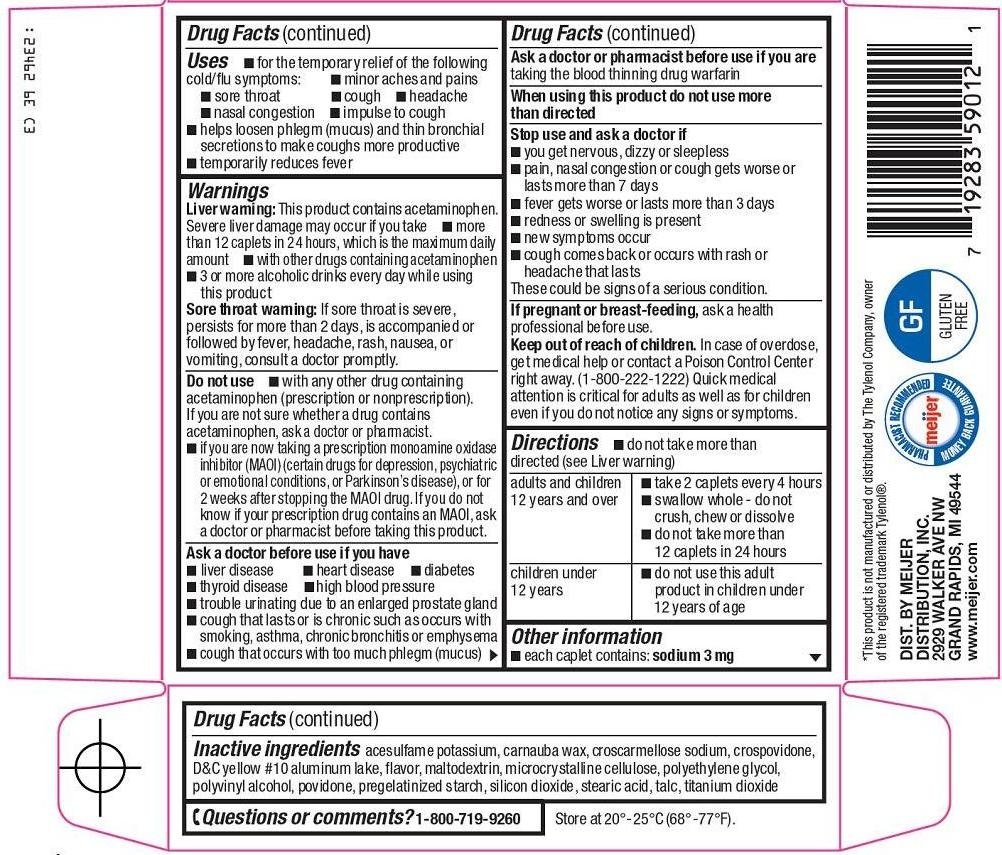
Uses
- for the temporary relief of the following cold/flu symptoms:
- minor aches and pains
- sore throat
- cough
- headache
- nasal congestion
- impulse to cough
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
taking the blood thinning drug warfarin
When using this product
do not use more than directed
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- pain, nasal congestion or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see Liver warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
- each caplet contains: sodium 3 mg
Inactive ingredients
acesulfame potassium, carnauba wax, croscarmellose sodium, crospovidone, D&C yellow #10 aluminum lake, flavor, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, pregelatinized starch, silicon dioxide, stearic acid, talc, titanium dioxide
Questions or comments?
1-800-719-9260
Principal Display Panel
Compare to Tylenol® Cold Head Congestion Severe active ingredients
See New Warnings Information
Cool Ice®
Instant Cooling Sensation
Cold Relief
Head Congestion
Severe
Pain Reliever/Fever Reducer, Nasal Decongestant, Cough Suppressant, Expectorant
Headache / Sore Throat – Acetaminophen
Nasal Congestion – Phenylephrine HCl
Coughing – Dextromethorphan HBr
Chest Congestion – Guaifenesin
Non-Drowsy
DayTime
Cold Relief Carton Image 1
Cold Relief Carton Image 2
| COLD RELIEF
acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 03/12/2008 | |
| Labeler - Meijer Distribution Inc (006959555) |