BIOELEMENTS IMMEDIATE COMFORT HYDROCORTISONE
-
hydrocortisone lotion
Bioelements, Inc.
----------
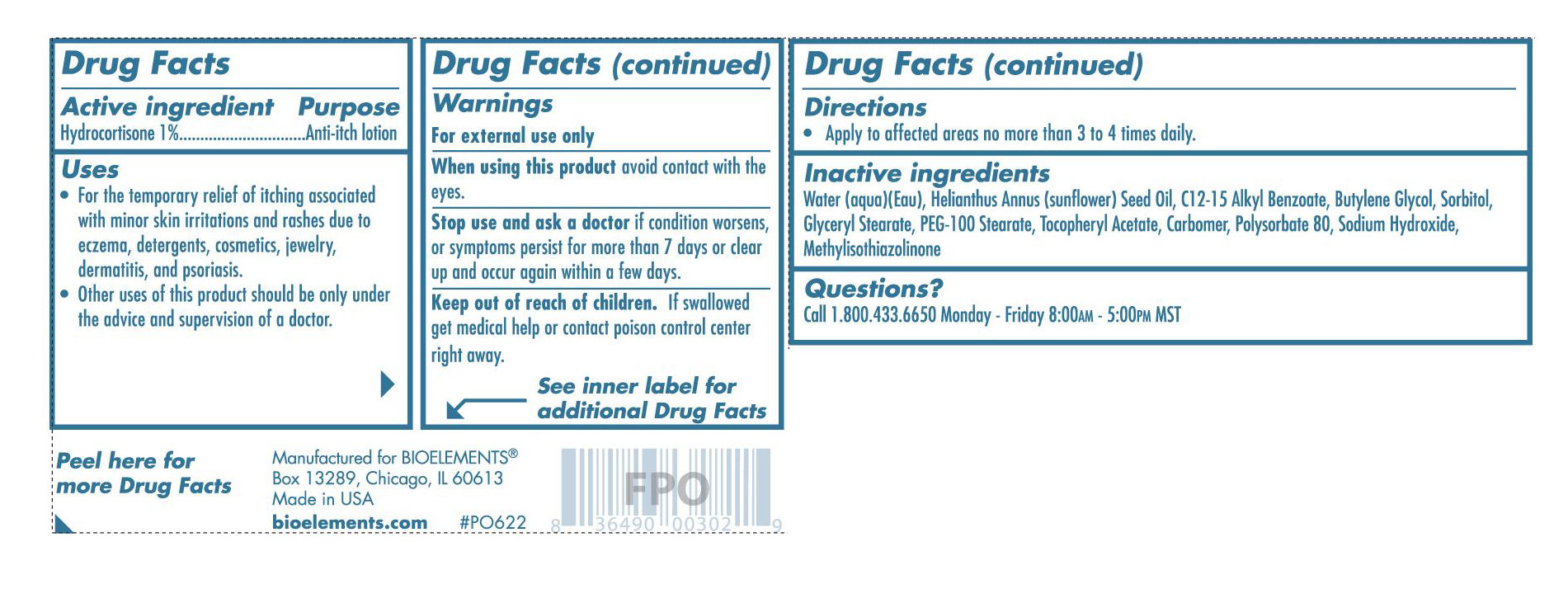
Immediate Comfort 1% Hydrocortisone Lotion
Active Ingredients Purpose
Hydrocortisone 1% Anti-Itch Lotion
for the temporary relief of itching associated with minor skin irritations and rashes due to eczema, detergents, cosmetics, jewelry, dermatitis, psoriasis.
-Keep out of reach of children. If swallowed get medical help or contact poison control center right away.
-For external use only
-When using this product avoid contact with the eyes-Stop use and ask a doctor if condition worsens, or symptoms persist for more than 7 days or clear up and occur again within a few days
-apply to affected areas no more than 3 to 4 times daily.
Inactive Ingredients: Water (Aqua) (Eau), Helianthus Annuus (Sunflower) Seed Oil, C12-15 Alkyl Benzoate, Butylene Glycol, Sorbitol, Glyceryl Stearate, PEG-100 Stearate, Tocopheryl Acetate, Carbomer, Polysorbate 80, Sodium Hydroxide, Methylisothiazolinone
Bioelements
Immediate Comfort - 1% Hydrocortisone Lotion
88 mL / 3 Fl. Oz.

| BIOELEMENTS
IMMEDIATE COMFORT HYDROCORTISONE
hydrocortisone lotion |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part348 | 08/19/2010 | |
| Labeler - Bioelements, Inc. (174813923) |
| Registrant - Bioelements, Inc. (174813923) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Chemolee Lab Corporation | 809982754 | manufacture | |