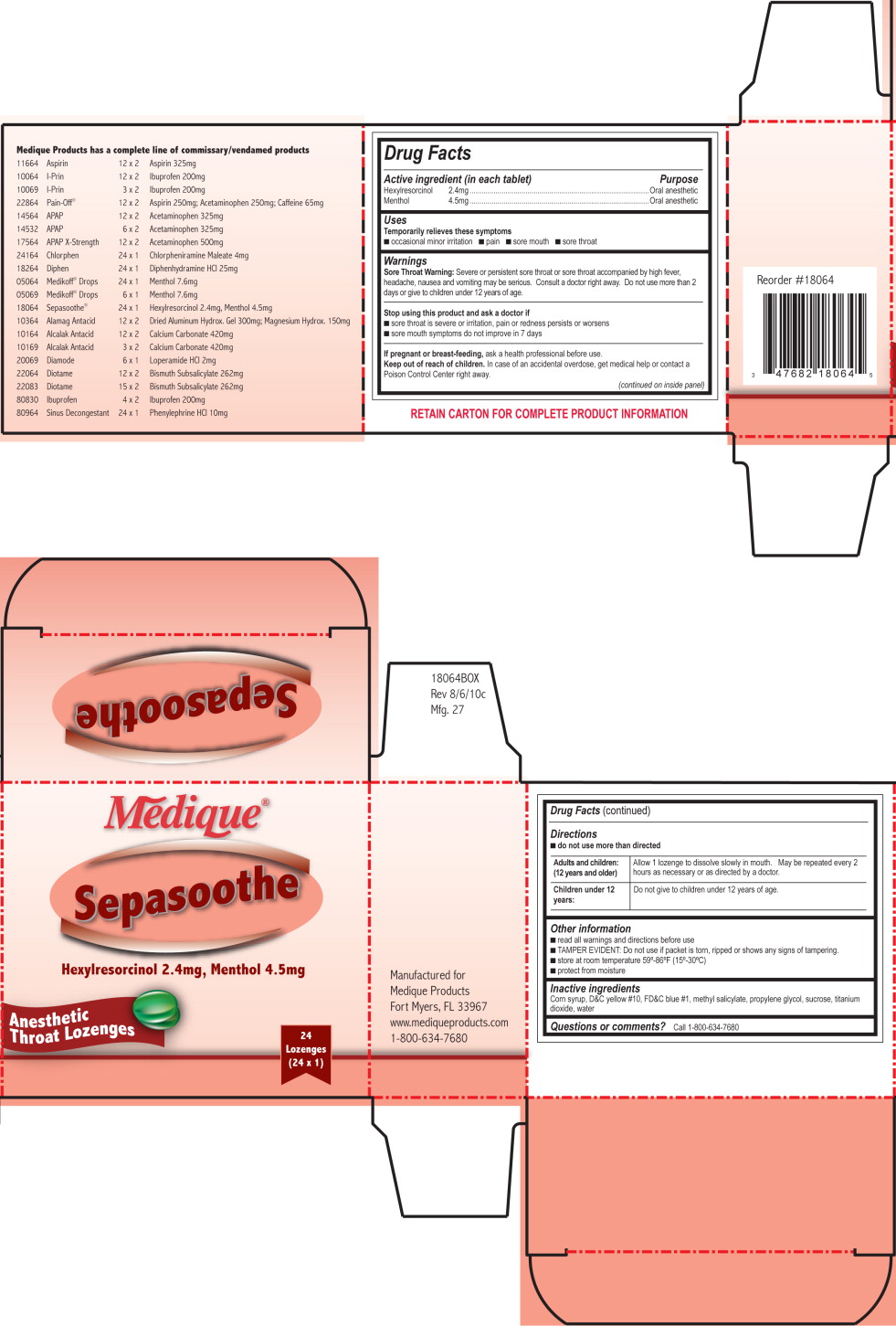
MEDIQUE SEPASOOTHE
-
hexylresorcinol and
menthol lozenge
Unifirst First Aid Corporation
----------
HEXYLRESORCINOL, MENTHOLDrug Facts
Active ingredient (in each lozenge)
Hexylresorcinol 2.4mg
Menthol 4.5mg
Purpose
Oral anesthetic
Oral anesthetic
Uses
Temporarily relieves these symptoms
- occasional minor irritation
- pain
- sore mouth
- sore throat
Warnings
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea and vomiting may be serious. Consult a doctor right away. Do not use more than 2 days or give to children under 12 years of age.
Stop using this product and ask a doctor if
- sore throat is severe or irritation, pain or redness persists or worsens
- sore mouth symptoms do not improve in 7 days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of an accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than directed
Adults and children: (12 years and older) Allow 1 lozenge to dissolve slowly in mouth. May be repeated every 2 hours as necessary or as directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age.
Other information
- read all warnings and directions before use.
- TAMPER EVIDENT: Do not use if packet is torn, ripped or shows any signs of tampering.
- store at room temperature 15º-30ºC (59º-86ºF)
- protect from moisture
Inactive ingredients
corn syrup, D&C yellow no. 10, FD&C blue no. 1, methyl salicylate, propylene glycol, sucrose, titanium dioxide, water
Questions or comments? Call 1-800-634-7680
909B Medique Sepasoothe Label
Medique®
Sepasoothe
Pull to Open
Hexylresorcinol 2.4 mg
Menthol 4.5 mg
Anesthetic
24 Lozenges
(24 x 1)
| MEDIQUE SEPASOOTHE
hexylresorcinol, menthol lozenge |
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part356 | 03/08/2011 | |
| Labeler - Unifirst First Aid Corporation (832947092) |