ALLERGY DN II
-
chlorpheniramine maleate and methscopolamine nitrate
Breckenridge Pharmaceutical, Inc.
----------
AllergyDN II™
Day Dose - 4 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
Night Dose - 8 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
DESCRIPTION
| Each Day tablet contains: | Each Night tablet contains: | ||
| Chlorpheniramine Maleate | 4 mg | Chlorpheniramine Maleate | 8 mg |
| Methscopolamine Nitrate | 2.5 mg | Methscopolamine Nitrate | 2.5 mg |
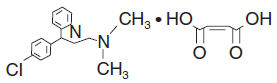
Chlorpheniramine maleate is an antihistamine having the chemical name 2-Pyridinepropanamine, γ-(4-chlorophenyl)-N, N-dimethyl-, (Z)-2-butenedioate (1:1); C16H19CIN2•C4H4O4, MW = 390.86.
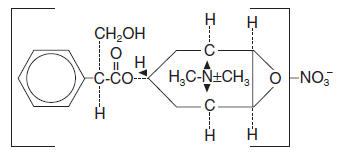
Methscopolamine nitrate is an anticholinergic having the chemical name 3-Oxa-9-azoniatricyclo [3.3.1.02,4] nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9, 9-dimethyl-, nitrate, [7(S)-(1α, 2β, 4β, 5α, 7β)]; C18H24NO4•NO3 MW = 380.40.
Inactive Ingredients:
Day tablets: Each beige Day tablet contains: Dicalcium Phosphate Dihydrate, Microcrystalline Cellulose, 1FD&C Yellow No. 6, FD&C Blue No. 2, Hypromellose, Silicon Dioxide, Croscarmellose Sodium, Magnesium Stearate.
Night tablets: Each green Night tablet contains: Dicalcium Phosphate Dihydrate, Microcrystalline Cellulose, 2FD&C Yellow No. 5 (tartrazine - see PRECAUTIONS), FD&C Blue No. 1, Hypromellose, Silicon Dioxide, Croscarmellose Sodium, Magnesium Stearate.
- 1
- Contains FD&C Yellow No. 6 as a color additive.
- 2
- This product contains FD&C Yellow No. 5 (tartrazine) as a color additive.
CLINICAL PHARMACOLOGY
Chlorpheniramine Maleate is an alkylamine-type antihistamine, which possesses anticholinergic and sedative effects. Antihistamines competitively antagonize histamine at the H1 receptor site. Thus, activation of H1 receptors by released histamine is prevented, resulting in increased vascular permeability and increased mucus production. Pruritus and sneezing are reduced.
Methscopolamine Nitrate is a quaternary ammonium derivative of the anticholinergic scopolamine which possesses the peripheral actions of the belladonna alkaloids, but does not exhibit the central actions because if its lack of ability to cross the blood-brain barrier. It competitively inhibits the action of acetylcholine at muscarinic receptors.
INDICATIONS AND USAGE
For the temporary relief of symptoms associated with allergic rhinitis.
CONTRAINDICATIONS
This product is contraindicated in patients with a hypersensitivity or idiosyncratic reaction to chlorpheniramine maleate or methscopolamine nitrate. This product is also contraindicated in nursing mothers, and in patients with the following conditions: severe coronary artery disease; narrow-angle glaucoma; urinary retention; hyperthyroidism; peptic ulcer; asthma attack; MAOI therapy (or for 2 weeks after stopping MAOI therapy).
WARNINGS
Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occurs, discontinue use and consult a doctor. If symptoms do not improve within 7 days or are accompanied by a fever, consult a doctor. Methscopolamine nitrate may produce dizziness or blurred vision. Patients taking this product should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous tasks while taking this drug. Alkylamine-type antihistamines should be used with extreme caution in patients with narrow-angle glaucoma; stenosing peptic ulcer; pyloroduodenal obstruction; symptomatic prostatic hypertrophy, or bladder neck obstruction. Due to its mild atropine-like action, chlorpheniramine should be used cautiously in patients with bronchial asthma, emphysema, or chronic pulmonary disease. May cause excitability, especially in children.
Heat prostration can occur with methscopolamine used where the environmental temperature is high. Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy; in this instance, use of methscopolamine would be inappropriate and possibly harmful.
Co-administration of sildenafil citrate and other organic nitrates has been shown to potentiate the hypotension effects of nitrates. Co-administration of Allergy DN II™ and sildenafil citrate has not been studied. Therefore, the use of sildenafil citrate and Allergy DN II™ together is not recommended.
PRECAUTIONS
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
General
Antihistamines have an atropine-like action and should be used with caution in patients with a history of bronchial asthma, emphysema, increased intraocular pressure, hyperthyroidism, cardiovascular disease and hypertension.
Use methscopolamine with caution in patients with hiatal hernia associated with reflux esophagitis. Use extreme caution and only when needed in patients with autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure, and cardiac arrhythmia. Investigate any tachycardia before giving any anticholinergic drugs since they may increase the heart rate. Prolonged use of anticholinergics may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort.
Drug Interactions
Concomitant use of antihistamines with alcohol, tricyclic antidepressants, barbiturates, and other CNS depressants may have an additive effect. MAO inhibitors (or for 14 days after stopping MAOI therapy) and tricyclic antidepressants may prolong and intensify the anticholinergic (drying) effects of antihistamines. Concomitant administration with antacids may interfere with the absorption of methscopolamine nitrate.
Information for Patients
Patient consultation should include the following information regarding proper use of this medication:
- Do not take more medication than the amount recommended.
- This medication should be used with caution during exercise or hot weather; overheating may result in heat stroke.
- Do not drive or operate machinery if drowsiness or dizziness occurs.
- Do not ingest alcoholic beverages, monoamine oxidase inhibitors (MAOI)s, or CNS depression producing medications (hypnotics, sedatives, tranquilizers) while taking this medication.
- Methscopolamine nitrate may cause blurred vision. Patients should observe caution before driving, using machinery or performing other tasks requiring visual alertness.
- If a dose is missed, the medication should be taken as soon as possible unless it is almost time for the next dose: do not double doses.
- Keep all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
Caution patients about the signs of potential side effects, especially:
- Anticholinergic effects - clumsiness or unsteadiness; severe drowsiness; severe dryness of mouth, nose, or throat; flushing or redness of face; shortness of breath or troubled breathing
- Blood dyscrasias - sore throat and fever; unusual bleeding or bruising; unusual tiredness or weakness
- Fast or irregular heartbeat
- Psychotic episodes
- Tightness in chest
Note: When anticholinergics are given to patients especially children, where the environmental temperature is high there is risk of a rapid increase in body temperature because of suppression of sweat gland activity. Infants, patients with Down's syndrome, and children with spastic paralysis or brain damage may show an increased response to anticholinergics, thus increasing the potential for side effects. Geriatric or debilitated patients may respond to usual doses of anticholinergics with excitement, agitation, drowsiness, or confusion.
Laboratory Tests
The following may be especially important in patient monitoring (other tests may be warranted in some patients, depending on conditions):
- Blood pressure determination - recommended at frequent intervals during therapy
- Electrocardiogram (ECG) - monitoring may be required
- Intraocular pressure determination - recommended at periodic intervals, as these medications may increase the intraocular pressure
Laboratory Test Interactions
Antihistamines may interfere with diagnostic test results for skin tests using allergen extracts. Anticholinergics may interfere with diagnostic test results for gastric acid secretion by antagonizing the effect of pentagastrin and histamine, and for radionucleotide gastric emptying studies by delaying gastric emptying.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies to assess the long-term carcinogenic and mutagenic potential or the effect on fertility in animals or humans have not been performed.
Pregnancy: Category C
Animal reproduction studies have not been conducted with this product. It is also not known whether is product can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
This product should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this combination product is excreted in human milk, and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or discontinue the product, taking into account the importance of the product to the mother.
Pediatric Use
The safety and effectiveness in children under 12 years of age have not been established.
Geriatric Use
The elderly (60 years and older) are more likely to experience adverse reactions to methscopolamine.
ADVERSE REACTIONS
Antihistamines and anticholinergics may cause drowsiness, dizziness, lassitude, blurred vision and excessive drying of the nose, throat and mouth, nausea, giddiness, increased irritability or excitement (especially in children).
OVERDOSAGE AND TREATMENT OF OVERDOSAGE
The treatment of overdosage should provide symptomatic and supportive care. Induction of emesis and gastric lavage may be performed if the patient is alert and seen within early hours after ingestion. Drug remaining in the stomach may be absorbed by the administration of activated charcoal. Since the effects of Allergy DN II™ last up to 12 hours, the patient should be monitored for at least that length of time and treated as necessary.
DOSAGE AND ADMINISTRATION
Adults and adolescents 12 years of age and over: One beige Day tablet in the morning and one green Night tablet in the evening. Allergy DN II™ is not recommended for children under 12 years of age.
HOW SUPPLIED
Allergy DN II™ is supplied in boxes of 20 tablets NDC #51991-534-20 One blister card of 10 Day Tablets NDC # 51991-533-17, and one blister card of 10 Night tablets NDC #51991-489-17.
Day tablets are beige, oval-shaped and scored, debossed with B 533.
Night tablets are green, oval-shaped and scored, debossed with B 489.
WARNING: Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
PHARMACIST: Dispense in original container.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) . See USP Controlled Room Temperature.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Manufactured by: Provident Pharmaceuticals, Colorado Springs, CO 80919
Distributed by: Breckenridge Pharmaceutical, Inc. Boca Raton, FL 33487
Rx Only
360672501 ISS. 4/08
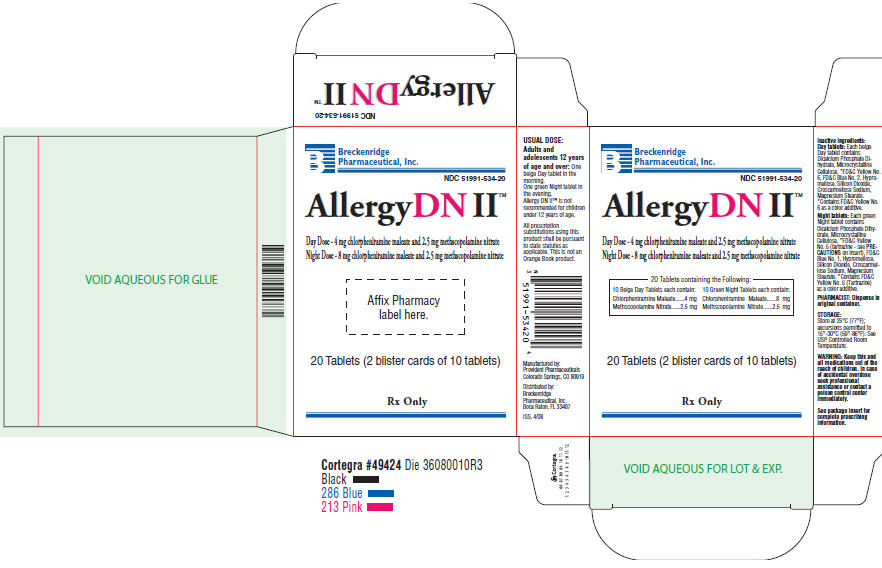
PRINCIPAL DISPLAY PANEL - 20 Tablet Carton
Breckenridge
Pharmaceutical, Inc.
NDC 51991-534-20
Allergy DN II™
Day Dose - 4 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
Night Dose - 8 mg chlorpheniramine maleate and 2.5 mg methscopolamine nitrate
20 Tablets containing the Following:
10 Beige Day Tablets each contain: 10 Green Night Tablets each contain:
Chlorpheniramine Maleate......4 mg Chlorpheniramine Maleate......8 mg
Methscopolamine Nitrate......2.5 mg Methscopolamine Nitrate......2.5 mg
20 Tablets (2 blister cards of 10 tablets)
Rx Only
| ALLERGY DN II
chlorpheniramine maleate and methscopolamine nitrate kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 11/01/2008 | 03/31/2010 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Provident Pharmaceuticals, LLC | 171901445 | MANUFACTURE | |