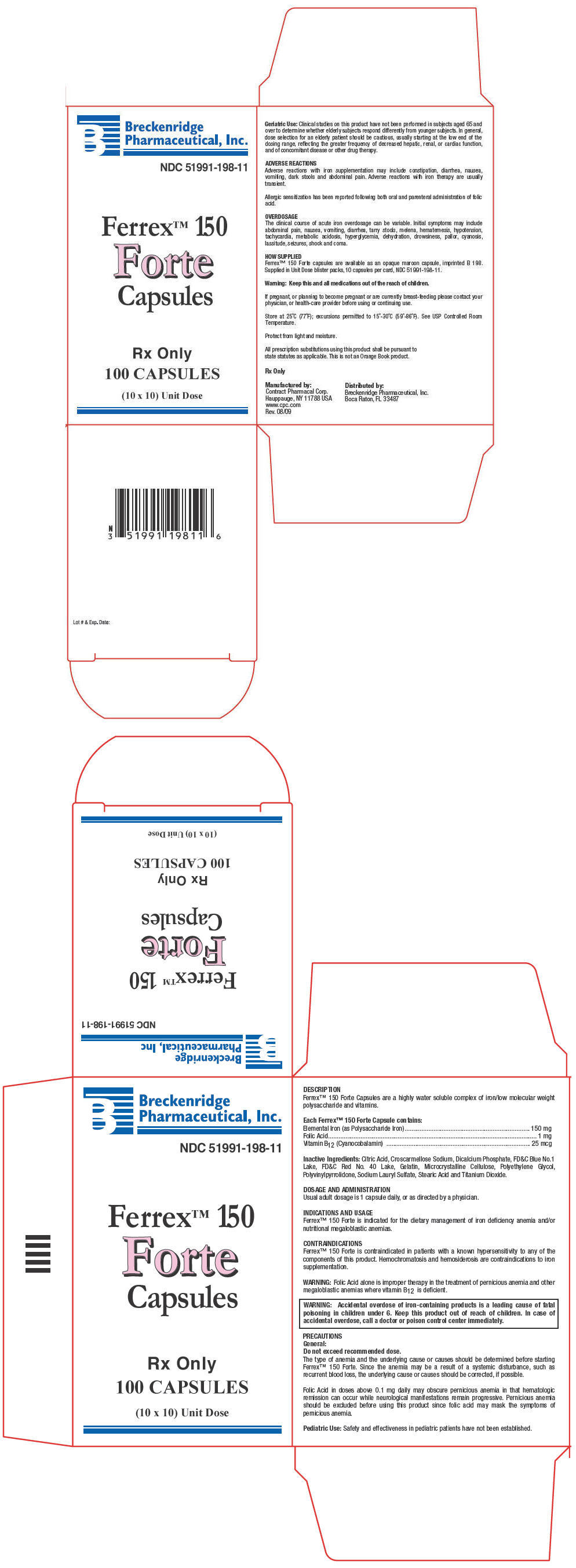
FERREX 150 FORTE
-
iron,
folic acid and
cyanocobalamin capsule
Breckenridge Pharmaceutical, Inc.
----------
Ferrex™ 150Forte
Capsules
DESCRIPTION
Ferrex™ 150 Forte Capsules are a highly water soluble complex of iron/low molecular weight polysaccharide and vitamins.
Each Ferrex™ 150 Forte Capsule contains:
| Elemental Iron (as Polysaccharide Iron) | 150 mg |
| Folic Acid | 1 mg |
| Vitamin B12 (Cyanocobalamin) | 25 mcg |
Inactive Ingredients: Citric Acid, Croscarmellose Sodium, Dicalcium Phosphate, FD&C Blue No.1 Lake, FD&C Red No. 40 Lake, Gelatin, Microcrystalline Cellulose, Polyethylene Glycol, Polyvinylpyrrolidone, Sodium Lauryl Sulfate, Stearic Acid and Titanium Dioxide.
DOSAGE AND ADMINISTRATION
Usual adult dosage is 1 capsule daily, or as directed by a physician.
INDICATIONS AND USAGE
Ferrex™ 150 Forte is indicated for the dietary management of iron deficiency anemia and/or nutritional megaloblastic anemias.
CONTRAINDICATIONS
Ferrex™ 150 Forte is contraindicated in patients with a known hypersensitivity to any of the components of this product. Hemochromatosis and hemosiderosis are contraindications to iron supplementation.
WARNING
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS
General
Do not exceed recommended dose.
The type of anemia and the underlying cause or causes should be determined before starting Ferrex™ 150 Forte. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. Pernicious anemia should be excluded before using this product since folic acid may mask the symptoms of pernicious anemia.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies on this product have not been performed in subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Adverse reactions with iron supplementation may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient.
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
HOW SUPPLIED
Ferrex™ 150 Forte capsules are available as an opaque maroon capsule, imprinted B 198. Supplied in Unit Dose blister packs, 10 capsules per card, NDC 51991-198-11.
Warning: Keep this and all medications out of the reach of children.
If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, or health-care provider before using or continuing use.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP Controlled Room Temperature.
Protect from light and moisture.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Rx Only
Manufactured by:
Contract Pharmacal Corp.
Hauppauge, NY 11788 USA
www.cpc.com
Rev. 08/09
Distributed by:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
PRINCIPAL DISPLAY PANEL - 100 Capsule Carton
Breckenridge
Pharmaceutical, Inc.
NDC 51991-198-11
Ferrex™ 150
Forte
Capsules
Rx Only
100 CAPSULES
(10 x 10) Unit Dose
| FERREX 150
FORTE
iron, folic acid, and cyanocobalamin capsule |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 04/01/2001 | 05/30/2012 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Contract Pharmacal Corp. | 057795122 | MANUFACTURE | |