LIDOCAINE HCL
-
lidocaine hydrochloride cream
Kylemore Pharmaceuticals, LLC
----------
Lidocaine HCl 3% CreamDESCRIPTION:
Contains lidocaine HCl 3% in a mild acidic vehicle. Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure:
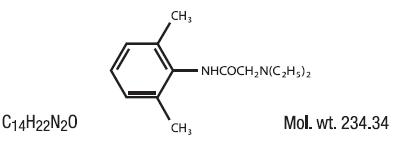
Each gram of Kylemmore Lidocaine HCl 3% Cream contains Lidocaine HCl USP 3%, Inactive Ingredients include: Purified Water, White Petrolatum USP, Stearic Acid NF, Cetyl Alcohol NF, Propylene Glycol USP, Stearyl Alcohol NF, Mineral Oil USP, Polysorbate 60, Sorbitan Monostearate, Methylparaben NF, Propylparaben NF, Sodium Hydroxide NF, Aluminum Sulfate and Calcium Acetate.
CLINICAL PHARMACOLOGY:
MECHANISM OF ACTION: Kylemmore Lidocaine HCl 3% releases lidocaine from a mild acidic vehicle to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. A mild acidic vehicle lowers pH to increase protection against alkaline irritations and to provide a favorable environment for healing.
PHARMACOKINETICS:
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver.
Lidocaine is metabolized rapidly by the liver and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/ toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline. The plasma binding of lidocaine is dependent on drug concentration and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein. Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion. Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics, but may increase the accumulation of metabolites. Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey, arterial blood levels of 18-21 g/mL have been shown to be threshold for convulsive activity.
INDICATIONS:
Anti-inflammatory anesthetic for relief of pruritus, pruritic eczemas, abrasions, minor burns, insect bites, pain, soreness and discomfort due to pruritus ani, pruritus vulvae, hemorrhoids, anal fissures, and similar conditions of the skin and mucous membranes.
CONTRAINDICATIONS:
Tuberculous or fungal lesions of skin vaccinia, varicella and acute herpes simplex and in persons who have shown hypersensitivity to any of its components. Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
WARNINGS:
For external use only. Not for ophthalmic use.
Keep product out of the reach of children.
PRECAUTIONS:
If irritation of sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. Kylemore Lidocaine HCl 3% Cream should be used with caution in ill, elderly, debilitated patients and children who may be more sensitive to the systemic effects of lidocaine.
CARCINOGENESIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY:
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
USE IN PREGNANCY:
Teratogenic Effects; Pregnancy Category B. Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing mother.
PEDIATRIC USE:
Dosage in pediatric patients would be reduced commensurate with age, body weight and physical condition.
ADVERSE REACTIONS:
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation.DOSAGE AND ADMINISTRATION:
Apply a thin film to the affected area two or three times daily or as directed by a physician.
HOW SUPPLIED:
Kylemmore Lidocaine HCl 3% Cream1 oz. (28.3 g) tube - NDC 49769-105-01
3 oz. (85 g) tube - NDC 49769-105-03
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at controlled room temperature 20º-25ºC (68º-77º F).
Protect from freezing.
Manufactured for:
Kylemore Pharmaceuticals
Port St. Joe, FL 32456
Iss. 12/09
105-10
PACKAGING:
Lidocaine HCl 3% Cream, 1 oz:

| LIDOCAINE HCL
lidocaine hydrochloride cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 12/01/2009 | 12/31/2012 | |
| Labeler - Kylemore Pharmaceuticals, LLC (831892471) |