BENPROX
-
benzoyl peroxide lotion
Kylemore Pharmaceuticals, LLC
----------
Benprox Wash 5.25%DESCRIPTION
Benprox Wash 5.25% is intended for topical administration which contain benzoyl peroxide for use in the treatment of acne vulgaris.
Active Ingredient: 5.25% Benzoyl Peroxide (containing dibenzoyl peroxide, water, and modifiers)
Inactive Ingredients: Allantoin, Aloe Barbadensis Leaf Juice, Benzyl Alcohol NF, Carbomer 940 NF, Disodium Laureth Sulfosuccinate, Glycerin, Panthenol, PPG-14 Palmeth-60 Hexyl Dicarbamate, Purified Water, and Triethanolamine. The structural formula of Benzoyl Peroxide is:
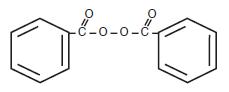
Benzoyl peroxide has a molecular weight of 242.23.
CLINICAL PHARMACOLOGY
Mechanism of Action
The exact mechanism of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl Peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action combined with the mild keralytic effect of Benzoyl Peroxide is believed to be responsible for its usefulness in acne. Benzoyl Peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS AND USAGE
Benprox Wash 5.25% is indicated for the topical treatment of acne vulgaris.
CONTRAINDICATION
Benprox Wash 5.25% is contraindicated in patients with a history of hyper-sensitivity to any of the ingredients in each product.
PRECAUTIONS
General: For external use only. If severe irritation develops, discontinue use and institute appropriate therapy. After reaction clears, treatment may often be resumed with less frequent application. These preparations should not be used in or near the eyes or on mucous membranes.
Patient Information and Cautions:
Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. Contact with any colored material (including hair and fabric) will result in bleaching or discoloration. If excessive irritation develops, discontinue use and consult your physician. When using this product, avoid unnecessary exposure to the sun and use a sunscreen.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Data from several studies employing a strain of mice that is highly susceptible to developing cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of these findings to humans is unknown. Benzoyl Peroxide has been found to be mutagenic (Ames Test) and there are no published data indicating it impairs fertility.
Pregnancy:
Teratogenic Effects: Pregnancy Category C: Animal reproduction studies have not been conducted with Benzoyl Peroxide. It is not known whether Benzoyl Peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl Peroxide should be used by a pregnant woman only if clearly needed. There are no available data on the effect of Benzoyl Peroxide on the later growth, development, and functional maturation of the unborn child.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Benzoyl Peroxide is administered to a nursing woman.
Pediatric Use: Safety and effectiveness in children under 12 years of age have not been established.
ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical Benzoyl Peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema, or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses maybe used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not an allergic reaction.
DOSAGE AND ADMINISTRATION
Benprox Wash 5.25% should be shaken well before use. The affected areas should be washed once or twice daily or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10 to 20 seconds, working into a full lather. Rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing cleanser off sooner or using the cleanser less often.
HOW SUPPLIED
Benprox Wash 5.25% is supplied in 175 g tubes, NDC 49769-199-58
Store at 20°-25°C (68°-77°F).Protect from freezing.
Rx Only
Manufactured for:
Kylemore Pharmaceuticals
Port St. Joe, FL 32456
Rev. 02/10 199-10 P0563
PACKAGING:
Below represents the current packaging being used:Tube:

Carton:

| BENPROX
benzoyl peroxide lotion |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 03/01/2010 | 03/16/2011 | |
| Labeler - Kylemore Pharmaceuticals, LLC (831892471) |