CLEAR EYES MAXIMUM ITCHY EYE RELIEF
-
glycerin,
naphazoline hydrochloride and
zinc sulfate liquid
Prestige Brands Holdings, Inc.
----------
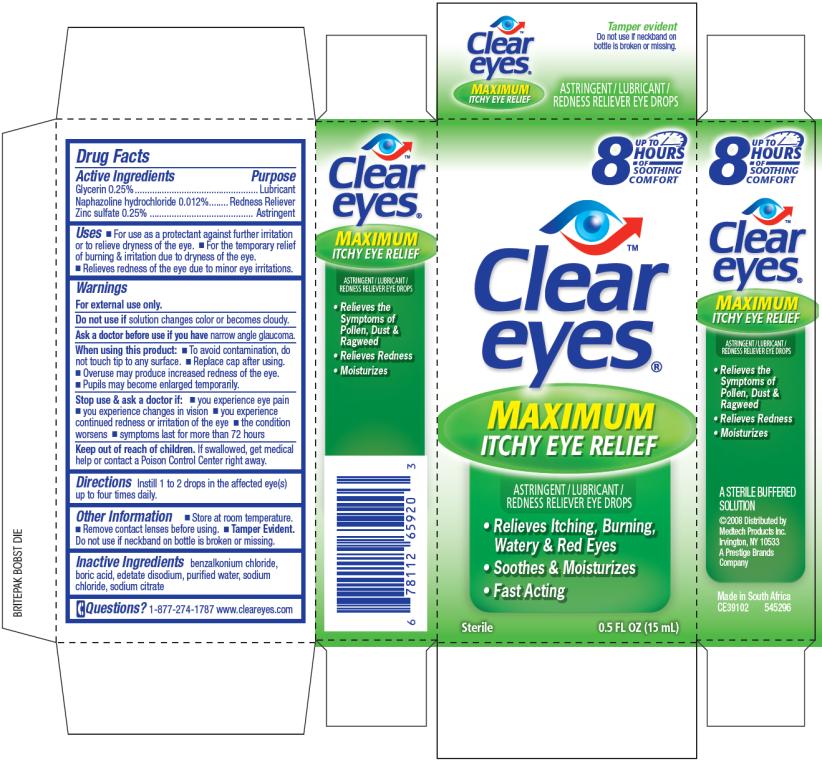
Clear Eyes Maximum Itchy Eye ReliefDrug Facts
Active Ingredient
Glycerin 0.25%
Purpose
Lubricant
Active Ingredient
Naphazoline hydrochloride 0.012%
Purpose
Redness Reliever
Active Ingredient
Zinc Sulfate 0.25%
Purpose
Astringent
Uses
- For use as a protectant against further irritation or to relieve dryness of the eye.
- For the temporary relief of burning & irritation due to dryness of the eye.
- Relieves redness of the eye due to minor eye irritations.
Warnings
For external use only.
Do not use if
solution changes color or becomes cloudy.
Ask a doctor before use if you have
narrow angle glaucoma.
When using this product
- To avoid contamination, do not touch tip to any surface.
- Replace cap after using.
- Overuse may produce increased redness of the eye.
- Pupils may become enlarged temporarily.
Stop use & ask a doctor if
- you experience eye pain
- you experience changes in vision
- you experience continued redness or irritation of the eye
- the condition worsens
- symptoms last for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Instill 1 to 2 drops in the affected eye(s) up to four times daily.
Other Information
- Store at room temperature.
- Remove contact lenses before using.
- Tamper Evident. Do not use if neckband on bottle is broken or missing.
Inactive Ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium chloride, sodium citrate
Questions?
1-877-274-1787 www.cleareyes.com
PRINCIPAL DISPLAY PANEL
UP TO 8 HOURS OF SOOTHING COMFORT
Clear eyes®
MAXIMUM ITCHY EYE RELIEF
ASTRINGENT/LUBRICANT/REDNESS RELIEVER EYE DROPS
● Relieves Itching, Burning, Watery & Red Eyes
● Soothes & Moisturizes
● Fast Acting
Sterile 0.5 FL OZ (15 mL)
| CLEAR EYES MAXIMUM ITCHY EYE RELIEF
glycerin and naphazoline hydrochloride and zinc sulfate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part349 | 03/15/2011 | |
| Labeler - Prestige Brands Holdings, Inc. (159655021) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aspen SVP (Pty) Ltd. | 538473091 | MANUFACTURE | |