DE LA CRUZ SULFUR ACNE MEDICATION
-
sulfur ointment
DLC Laboratories, Inc.
----------
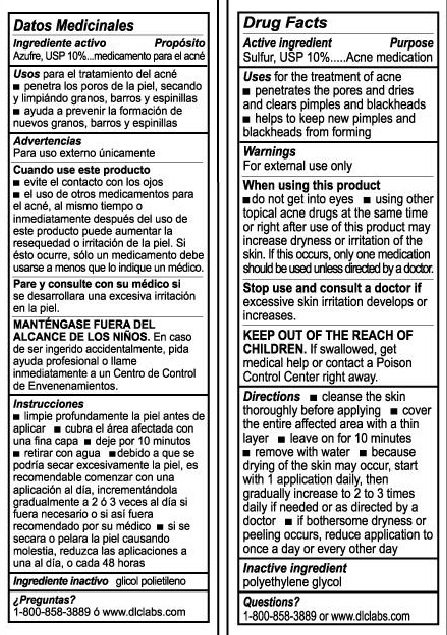
De La Cruz Sulfur Ointment 10% Acne MedicationActive Ingredient
Sulfur USP, 10%
Purpose
Acne Medication
Uses
for the treatment of acne
penetrates the pores and dries and clears pimples and blackheads
helps to keep new pimples and blackheads from forming.
Warnings
for external use only
When using this product
do not get into eyes
using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin.
If this occurs, only one medication should be used unless directed by a doctor
Stop use and consult a doctor if
excessive skin irritation develops or increases
KEEP OUT OF THE REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away
Directions
cleanse the skin thoroughly before applying
cover the entire affected area with a thin layer
leave on for 10 minutes
remove with water
because drying of the skin my occur, start with 1 application daily, then gradually increase to 2 to 3 times daily if needed or as directed by a doctor.
if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
polyethylene glycol
Questions?
1-800-858-3889 or www.dlclabs.com
De La Cruz
SULFUR Ointment 10%
Acne Medication
2.6 ox (73.7g)
Dries and clears pimples and blackheads
Helps to keep new pimples and blackheads from forming
Oil-free, water washable base
No preservatives, artificial colors or fragrances
Manufactured by
De La Cruz Products
A Division of DLC Laboratories, Inc.
Paramount, CA 90723 USA
Questions: 1-800-858-3989
www.dlclabs.com (c) DLC

| DE LA CRUZ SULFUR ACNE MEDICATION
sulfur ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333D | 02/09/2011 | |
| Labeler - DLC Laboratories, Inc. (093351930) |