CORZALL PE
-
carbetapentane citrate,
phenylephrine hydrochloride and
dexchlorpheniramine maleate liquid
Hawthorn Pharmaceuticals, Inc.
----------
CORZALL-PE LiquidCORZALL-PE Liquid
Rx OnlyDESCRIPTION:
Each teaspoonful (5 mL) for oral administration contains:
Carbetapentane Citrate.......................................................20 mg
Phenylephrine Hydrochloride...............................................10 mg
Dexchlorpheniramine Maleate...............................................1 mg
Inactive ingredients: citric acid, sodium citrate, sodium saccharine, cherry flavor, propylene glycol, sorbitol, glycerine, and purified water.
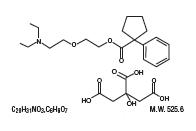
Carbetapentane Citrate (1-Phenylcyclopentanecarboxylic acid, 2-(2-diethylaminoethoxy) ethyl ester citrate) is a white crystalline powder. It is freely soluble in water and chloroform. Its structure is as follows:
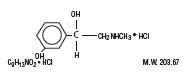
Phenylephrine hydrochloride is a sympathomimetic amine used as a vasoconstrictor having the name (-) -m-Hydroxy-a-[(methylamino)methyl]benzyl alcohol hydrochloride. Its chemical structure is as follows:
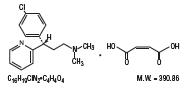
Dexchlorpheniramine maleate is an antihistamine having the chemical name (+)-2-[p-Chloro-a-[2-dimethylamino)ethyl]benzyl]pyridine maleate (1:1). Its structural formula is as follows:
CLINICAL PHARMACOLOGY
Antitussive, decongestant, and antihistaminic actions.Carbetapentane citrate is a centrally acting non-narcotic antitussive. Carbetapentane citrate has atropine-like and local anesthetic actions, as well as temporarily controls and suppresses the cough reflex by selective depression of the medullary cough center. It has no significant analgesic or sedative properties, does not depress respiration or predispose to addiction with usual doses.
Phenylephrine hydrochloride is primarily a direct-acting sympathomimetic amine; however, it also has some indirect action by releasing norepinephrine from its storage sites. Phenylephrine acts on alpha-adrenergic receptors to produce vasoconstriction which increases peripheral resistance, resulting in an increase in both systolic and diastolic blood pressure. Accompanying the pressor response to phenylephrine is a marked reflex bradycardia due to increased vagal activity. In therapeutic doses, phenylephrine hydrochloride produces little or no central nervous system (CNS) stimulation. Following oral administration, constriction of blood vessels in the nasal mucosa may relive nasal congestion.
Dexchlorpheniramine maleate is an antihistamine with anticholinergic properties. It is capable of producing a slight to moderate sedative effect. Antihistamines appear to compete with histamine for receptor sites on effector cells and are of value clinically in the prevention and relief of many allergic manifestations. In vitro and in vivo assays of the antihistamine chlorpheniramine demostrate that the predominant activity is in the dextro-isomer. The dextro-isomer is approximately tow times more active than the racemic compound. Since dexchlorpheniramine is the dextro-isomer and active moiety of chlorphenirmaine, it can be assumed that experiences with chlorpheniramine also apply to dexchlorpheniramine. Dexchlorpheniramine maleate competitively antagonizes most of the smooth muscle stimulating actions of histmine on the H1receptors and the GI tract, uterus, large blood vessels and bronchial muscle. It also antagonizes the action of histamine that results in increased capillary permeablity and the formation of edema.
INDICATIONS AND USAGE
CorzallTM-PE Liquid is indicated for the symptomatic relief of the cough, coryza and nasal congestion associated with the common cold, sinusitis, allergic rhinitis and other upper respiratory tract conditions. Appropriate therapy should be provided for the primary disease.CONTRAINDICATIONS
Patients with hypersensitivity or idiosyncrasy to any of its ingredients. Sympathomimetic amines are contraindicated in patients with sever hypertension, sever coronary heart disease and patients on monoamine oxidase (MAO) inhibitor therapy or for two weeks after stopping the MAOI drug. Antihistamines are contraindicated in patients with narrow-angle glaucoma, urinary retention, peptic ulcer, and during an asthma attack.WARNINGS
Sympathomimetic amines should be used judiciously and sparingly in patients with hypertension, diabetes, ischemic heart disease, hyperthyroidism, increased intraocular pressure, or prostatic hypertrophy. (See CONTRAINDICATIONS). Sympathomimetic amines may produce CNS stimulation with convulsions or cardiovascular collapse with accompanying hypotention. The elderly (60 years and older) are more likely to exhibit adverse reactions.A persistent cough may be a sigh of a serious condition. If cough persists for more than one week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor. Patient re-evaluation should be considered.
Antihistamines may cause hyperexcitability, especially in children. At doses higher than the recommended doses, nervousness, dizziness or sleeplessness may occur. Do not exceed recommended dosage.
PRECAUTIONS
General: There are studies which demonstrate that carbetapentane has antitussive activity which may suppress or modify cough. Before prescribing medication to suppress or modify cough, identify and provide therapy for the underlying cause of the cough and take caution that modification of cough does not increase the risk of clinical or physiologic complications.Use with caution in patients with hypertension, heart disease, asthma, hyperthyroidism, increased intraocular pressure, diabetes mellitus, and prostatic hypertrophy.
Antihistamines are more likely to cause dizziness, sedation and hypotension in elderly patients. Antihistamines may cause excitation, particularly in children, but their combination with sympathomimetics may cause either mild stimulation or mild sedation.
Information for patients:
Avoid alcohol and other CNS depressants while taking this product. Patients sensitive to antihistamines may experience moderate to severe drowsiness. Patients sensitive to sympathomimetics may note mild CNS stimulation.Antihistamines may impair mental and physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. Patients should be warned accordingly.
Drug Interactions:
Carbetapentane citrate should not be used in patients receiving MAO inhibitors, including 14 days after stopping the MAOI drug. The use of carbetapentane citrate may result in additive CNS depressant effects when coadministered with alcohol, antihistamines, phychotropics or other drugs that produce CNS depression.Antihistamines may enhance the effect of tricyclic antidepressants, barbiturates, alcohol, and other CNS depressants. MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines.
Sympathomimetic amines may reduce the antihypertensive effects of reserpine, vertrum alkaloids, methyldopa, and mecamylamine. Effects of sympathomimetics are increased with MAO inhibitors and beta-adrenergic blockers.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term animal studies have been performed with CorzallTM-PE Liquid.Pregnancy:
Pregnancy Category C: Animal reproduction studies have not been conducted with this product. it is not known whether this product can cause fetal harm when administered to a pregnant woman or affect reproductive capacity. Give to pregnant women only if clearly needed. Dexchlorphenirmine maleate should not be used in the third trimester of pregnancy because newborn and premature infants may have severe reaction to antihistamines.Nursing Mothers:
It is not known if the drugs in this product are excreted in human milk. Since many drugs may be excreted into human milk and because of the potential for serious side effects in the nursing infant, this product should only be given to nursing mothers if clearly needed.Pediatric Use:
Safety and effectiveness in pediatric patients below the age of six have not been established. Product not intended for administration for children 6 years of age and under.Geriatric Use:
The elderly (60 years and older) are more likely to have adverse reactions to sympathomimetics. Overdosage of sympathomimetics in this age group may cause hallucinations, convulsions, CNS depression and death.Carbetapentane Citrate: Adverse effects associated with carbetapentane citrate are rare, but nausea and/or other gastrointestinal disturbances sometimes occur.
Phenylephrine Hydrochloride: Sympathomimetics may cause mild central nervous system stimulation, especially in those who are hypersensitive to sympathomimetic drugs. Nervousness, excitability, restlessness, dizziness, weakness and insomnia may also occur. Headache and drowsiness have also been reported. Sympathomimetic drugs have also been associated with certain untoward reactions including fear, anxiety, tenseness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, CNS stimulation, nervousness, increased heart rate or blood pressure, arrhythmias and cardiovascular collapse with hypotension.
Dexchlorpheniramine Maleate: Slight to moderate drowsiness may occur and is the most frequent side effect. Sedation, dizziness, diplopia, vomiting, diarrhea, dry mouth, headache, nervousness, nausea, anorexia, heartburn, weakness, polyuria, and dysuria, and rarely, excitability in children. Urinary retention may occur in patients with prostatic hypertrophy.
OVERDOSAGE
The signs, symptoms and treatment described below are those of H1 antihistamine, phenylephrine and carbetapentaine.Symptoms: Should antihistamine effects predominate, central action constitutes the greatest danger. In the small child, predominate symptoms are excitation, hallucination, ataxia, incoordination, tremors, flushed face and fever. Convulsions, fixed and dilated pupils, coma, and death may occur in severe cases. In the adult, fever and flushing are uncommon; excitement leading to convulsions and postictal depression is often preceded by drowsiness and coma. Respiration is usually not seriously depressed; blood pressure is usually stable.
Should sympathomimetic symptoms predominate, central effects include restlessness, dizziness, tremor, hyperactive reflexes, talkativeness, irrability, and insomnia. Cardiovascular and renal effects include difficulty in micturition, headache, flushing, palpitation, cardiac arrhythmia, hypertension with subsequent hypotension and circulatory collapse. Gastrointestinal effects include dry mouth, metallic taste, anorexia, nausea, vomiting, diarrhea and abdominal cramps.
Treatment: The stomach should be emptied promptly by emetics and/or gastric lavage. The installation of activated charcoal also should be considered. Cardiac function and serum electrolytes should be monitored and treatment initiated if indicated. If convulsions or marked CNS excitement occurs, diazepam may be used.
DOSAGE AND ADMINISTRATION*
Adults and children 12 years of age and older: 1-2 teaspoonfuls (5-10 mL) every 4-6 hours, not to exceed 6 teaspoonfuls in a 24 hour period.Children 6 to 12 years of age: 1/2-1 teaspoonfuls (2.5-5 mL) every 4-6 hours, not to exceed 3 teaspoonfuls in a 24 hour period.
Children under 6 years of age: Consult a physician.
*In mild cases or in particularly sensitive patients, less frequent or reduced doses may be adequate.
HOW SUPPLIED
CorzallTM-PE Liquid is supplied as a clear, cherry flavored liquid available in 16 fl oz bottles, NDC 63717-556-16 and 1/2 fl oz (15 mL) sample bottles, NDC 63717-556-99.KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]
Dispense in a tight, light-resistant container as defined in the USP/NF with a child-resistant closure.
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
Manufactured for:
Hawthorn Pharmaceuticals, Inc.
Madison, MS 39110
HI 260 08/10
Product Packaging:
The packaging below represents labeling currently used.Principal display panel and side panel for 473 mL label:
NDC 63717-556-16
CORZALLTM-PE LIQUID
Antitussive/ Decongestant/ Antihistamine
Each teaspoonful (5 mL) for oral administration contains:
Carbetapentane Citrate.....................................20 mg
Phenylephrine HCl............................................10 mg
Dexchlorpheniramine Maleate..............................1 mg
Rx Only
Dye Free/ Sugar Free/ Alcohol Free
HAWTHORN PHARMACEUTICALS, INC.
16 fl oz (473 mL)
USUAL DOSAGE: See Package Insert for Complete Dosage Recommendations.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant closure.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 (Toll Free).
Manufactured for: Hawthorn Pharmaceuticals, Inc., Madison, MS 39110
HL217 08/10



| CORZALL
PE
carbetapentane citrate, phenylephrine hydrochloride, dexchlorpheniramine maleate liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 09/22/2010 | ||
| Labeler - Hawthorn Pharmaceuticals, Inc. (118049704) |