lymphazurin (isosulfan blue) injection, solution
[United States Surgical, a division of Tyco Healthcare Group LP]
(isosulfan blue) in aqueous solution for subcutaneous administration
10000-29690
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
IMPORTANT!
This booklet is designed to assist in using this product. It is not a reference to surgical techniques.
This device was designed, tested and manufactured for single patient use only. Reuse or reprocessing of this device may lead to its failure and subsequent patient injury. Reprocessing and/or resterilization of this device may create the risk of contamination and patient infection. Do not reuse, reprocess or resterilize this device.
DESCRIPTION
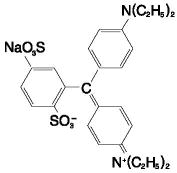
The chemical name for LYMPHAZURIN™ 1% (isosulfan blue) is N-[4-[[4-(diethylamino)phenyl] (2,5-disulfophenyl) methylene]-2,5-cyclohexadien-1-ylidene]-N-ethylethanaminium hydroxide, inner salt, sodium salt. Its structural formula is:
LYMPHAZURIN™ 1% (isosulfan blue) is a sterile aqueous solution for subcutaneous administration. Phosphate buffer in sterile, pyrogen-free water is added in sufficient quantity to yield a final pH of 6.8-7.4. Each ml of solution contains 10 mg isosulfan blue, 6.6 mg sodium monohydrogen phosphate and 2.7 mg potassium dihydrogen phosphate. The solution contains no preservative.
LYMPHAZURIN™ 1% (isosulfan blue) is a contrast agent for the delineation of lymphatic vessels.
INDICATIONS
LYMPHAZURIN™ 1% (isosulfan blue) upon subcutaneous administration, delineates the lymphatic vessels. It is an adjunct to lymphography (in primary and secondary lymphedema of the extremities; chyluria, chylous ascites or chylothorax; lymph node involvement by primary or secondary neoplasm; and lymph node response to therapeutic modalities) for visualization of the lymphatic system draining the region of injection.
CLINICAL PHARMACOLOGY
LYMPHAZURIN™ 1% (isosulfan blue) has no known pharmacologic action. Following subcutaneous administration, isosulfan blue is selectively picked up by the lymphatic vessels. Thus, the lymphatic vessels are delineated by a bright blue color making them discernible from surrounding tissue.
There is some evidence that 50% of isosulfan blue, from aqueous solution, is weakly bound to serum protein (albumin). Since interstitial protein is presumed to be carried almost exclusively by lymphatics, and in view of evidence of binding of dyes to proteins, visualization may be due to protein binding phenomenon.
ABSORPTION
Following a single 1 ml subcutaneous injection of a 1% solution by triphenylmethane dye in the rat, 34% is absorbed in 30 minutes from the injection site. Absorption of 69% and 100% occurs at 1 and 24 hours respectively.
EXCRETION
Up to 10% of the subcutaneously administered dose of LYMPHAZURIN™ 1% (isosulfan blue) is excreted unchanged in the urine in 24 hours in man. Presumably, 90% is excreted through the biliary route.
CONTRAINDICATIONS
LYMPHAZURIN™ 1% (isosulfan blue) is contraindicated in those individuals with known hypersensitivity to triphenylmethane or related compounds.
WARNINGS
1. The lymphographic procedure which involves the use of LYMPHAZURIN™ 1% (isosulfan blue) should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the procedure to be performed. Appropriate facilities should be available for coping with situations which may arise as a result of the procedure, as well as for emergency treatment of severe reactions to the drug.
2. After subcutaneous administration of LYMPHAZURIN™ 1% (isosulfan blue), competent personnel and emergency facilities should be available for at least 30 to 60 minutes, since severe delayed reactions have been known to occur with similar compounds.
3. The admixture of LYMPHAZURIN™ 1% (isosulfan blue) with local anesthetics (i.e., lidocaine) in the same syringe prior to administration results in an immediate precipitation of 4-9% drug complex. This technique is not recommended. If it is in the best interest of the patient to give a local anesthetic, it is suggested that administration be made via a separate syringe.
PRECAUTIONS
INFORMATION FOR PATIENTS
Since up to 10% of LYMPHAZURIN™ 1% (isosulfan blue) is excreted unchanged in the urine, the patient should be advised that urine color may be blue for 24 hours following its administration.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of LYMPHAZURIN™ 1% (isosulfan blue) and are, therefore, unknown. Similarly, reproduction studies in animals have not been conducted and, therefore, it is unknown if a problem concerning mutagenesis or impairment of fertility in either males or females exists.
TERATOGENIC EFFECTS
Pregnancy Category C: Animal reproduction studies have not been conducted with LYMPHAZURIN™ 1% (isosulfan blue). It is also not known whether LYMPHAZURIN™ 1% (isosulfan blue) can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. LYMPHAZURIN™ 1% (isosulfan blue) should be given to a pregnant woman only if clearly needed.
NURSNG MOTHERS
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LYMPHAZURIN™ 1% (isosulfan blue) is administered to a nursing mother.
PEDIATRIC USE
Safety and effectiveness of LYMPHAZURIN™ 1% (isosulfan blue) in children have not been established.
ADVERSE REACTIONS
1. LYMPHAZURIN™ 1% (isosulfan blue) has demonstrated a 1.5% incidence of adverse reactions. All the reactions were of an allergic type. Localized swelling at the site of administration and mild pruritis of hands, abdomen and neck have been reported within several minutes following administration of the drug.
2. Reports of mild to severe reactions have appeared in the literature for compounds similar to isosulfan blue. A death has been reported following the intravenous administration of a similar compound employed to estimate depth of a severe burn. Severe reactions may be manifested by edema of the face and glottis, respiratory distress or shock; such reactions may prove fatal unless promptly controlled by such emergency measures as maintenance of a clear airway and immediate use of oxygen and resuscitative drugs. Like other sensitivity phenomena, severe reactions are more likely to occur in patients with a personal or family history of bronchial asthma, significant allergies, drug reactions or previous reactions to triphenylmethane dyes.
DOSAGE AND ADMINISTRATION
LYMPHAZURIN™ 1% (isosulfan blue) is to be administered subcutaneously, one-half (1/2) ml into three (3) interdigital spaces of each extremity per study. A maximum dose of 3 ml (30 mg) isosulfan blue is, therefore, injected.
HOW SUPPLIED
LYMPHAZURIN™ 1% (isosulfan blue) is supplied as a 5 ml single dose vial, 1% aqueous solution in a phosphate buffer prepared by appropriate manufacturing to be sterile and pyrogen-free.
STORE AT ROOM TEMPERATURE. AVOID PROLONGED EXPOSURE TO ELEVATED TEMPERATURES.
NDC 63261-250-21
Establishment License #: 100689-A
Control #: 081375
D.I.N. 00592358
Manufactured by: United States Surgical, a division of Tyco Healthcare Group LP, Norwalk, CT 06856 USA.
© 2005 United States Surgical, a division of Tyco Healthcare Group LP.
All Rights Reserved. 7/05 -4
By: Ben Venue Labs., Inc., Bedford, OH 44146 Distributed in Canada by: Tyco Healthcare Montreal, Quebec, Canada H9R 5H8
| Lymphazurin (isosulfan blue) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 12/2006United States Surgical, a division of Tyco Healthcare Group LP