MEDACTIVE PATIENT FRIENDLY
-
stannous fluoride
MedActive Oral Pharmaceuticals, LLC.
----------
Drug Facts
Active Ingredient
Stannous Fluoride 0.63%
Purpose
Remineralizes and strengthens tooth surfaces and aids in the prevention of dental decay.
Keep out of the reach of children under age 6.
- If more than used for rinsing is accidentally swallowed, seek medical attention or contact a Poison Control Center immediately.
Uses
- Remineralizes and strengths tooth surface.
- Cleans, lubricates, moistens and protects oral cavity.
- Aids in the prevention of dental decay.
- Builds increasing protection against tooth sensitivity.
Other Information
- This product may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
- Do not refrigerate. Store at room temperature.
- See box for expiration date.
Directions
- Use immediately after preparing the rinse.
For adults and children 6 years of age and older:
- Place both thumbs on Blue Chamber No. 1 and Press down firmly to squeeze fluoride into Chamber No. 2.
- Gently Shake.
- Snap Tab to Open by bending at line on cap. Dispense half of mixture into mouth and Rinse for 30 seconds. Spit.
- Gargle with remainder of mixture for 30 seconds. Spit.
- Avoid any food or drinks for at least 15 minutes after use.
For children under 6 years of age: consult a dentist or doctor.
Inactive Ingredients
Water, Sorbitol, Glycerin, ULTRAMULSION®, Potassium Sorbate, EDTA, Sucralose, Flavor.

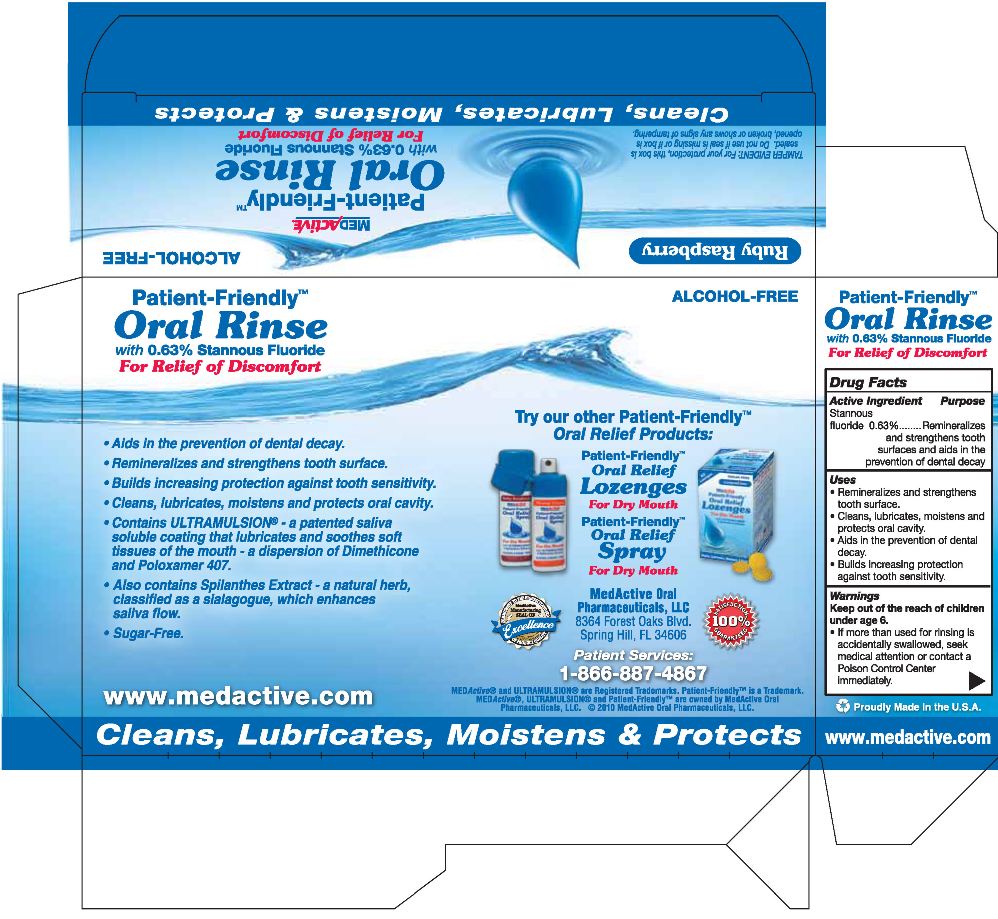
Ruby Raspberry with ULTRAMULSION® and Spilanthes Extract
ALCOHOL-FREE
MEDActive®
Patient-Friendly™
Oral Rinse
with 0.63% Stannous Fluoride
For Relief of Discomfort
IMPORTANT: Read directions for proper use. Net Contents: 10 Dose Packs, 10.4 FL OZ (307 mL)
MEDACTIVE PATIENT FRIENDLY
stannous fluoride
kit |
|
|
|
|
|
|
| Part 1 of 2 |
CHAMBER NO. 1
stannous fluoride
concentrate |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 2 |
CHAMBER NO. 2
stannous fluoride diluent
emulsion |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revised: 03/2010MedActive Oral Pharmaceuticals, LLC.

