CLARITIN
-
loratadine solution
Schering-Plough HealthCare Products, Inc.
----------
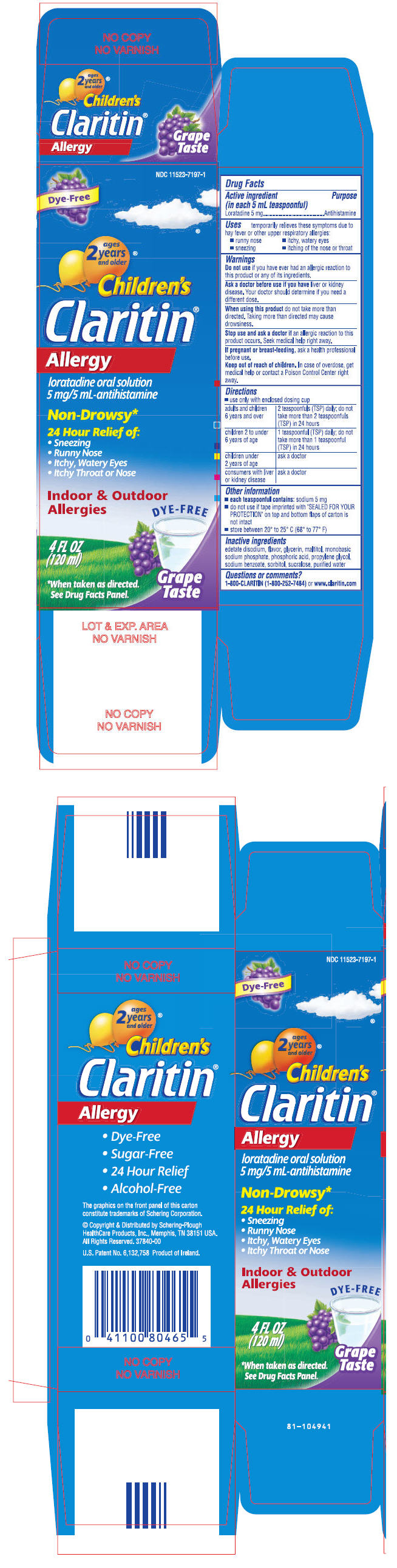
CHILDREN'S CLARITIN –loratadineActive ingredient (in each 5 mL teaspoonful)
Loratadine 5 mg
Purpose
Antihistamine
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Use only with enclosed dosing cup
- adults and children 6 years and over: 2 teaspoonfuls (TSP) daily; do not take more than 2 teaspoonfuls (TSP) in 24 hours
- children 2 to under 6 years of age: 1 teaspoonful (TSP) daily; do not take more than 1 teaspoonful (TSP) in 24 hours
- children under 2 years of age: ask a doctor
- consumers with liver or kidney disease: ask a doctor
Other information
- each teaspoonful contains: sodium 5 mg
- do not use if tape imprinted with "SEALED FOR YOUR PROTECTION" on top and bottom flaps of carton is not intact.
- store between 20° and 25°C (68° and 77°F)
Inactive ingredients
edetate disodium, flavor, glycerin, maltitol, monobasic sodium phosphate, phosphoric acid, propylene glycol, sodium benzoate, sorbitol, sucralose, purified water
Questions or comments?
1-800-CLARITIN (1-800-252-7484) or www.claritin.com
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
NDC 11523-7197-1
Dye-Free
ages
2 years
and older®
Children's
Claritin®
Allergy
loratadine oral solution
5 mg/5 mL-antihistamine
Non-Drowsy*
24 Hour Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Indoor & Outdoor
Allergies
DYE-FREE
4 FL OZ
(120 ml)
Grape
Taste
*When taken as directed.
See Drug Facts Panel.
| CLARITIN
loratadine solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA020641 | 03/01/2011 | |
| Labeler - Schering-Plough HealthCare Products, Inc. (039137567) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Schering-Plough Canada | 207093332 | Manufacture | |