GUNA-INF ALPHA
-
interferon alfa-2b solution/ drops
Guna spa
----------
DRUG FACTSACTIVE INGREDIENT
INTERFERON ALFA-2B 4C
PURPOSE
RECURRENT VIRAL INFECTIONS
KEEP OUT OF REACH OF CHILDREN
Keep this and all medicines out of reach of children
INDICATIONS & USAGE
RECURRENT VIRAL INFECTIONS
WARNINGS
Stop use and ask doctor if symptoms worsen or persist more than 3 days
DIRECTIONS
Take 15 minutes before meals.
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.
INACTIVE INGREDIENT
Ethyl Alcohol 30%
PRINCIPAL DISPLAY PANEL
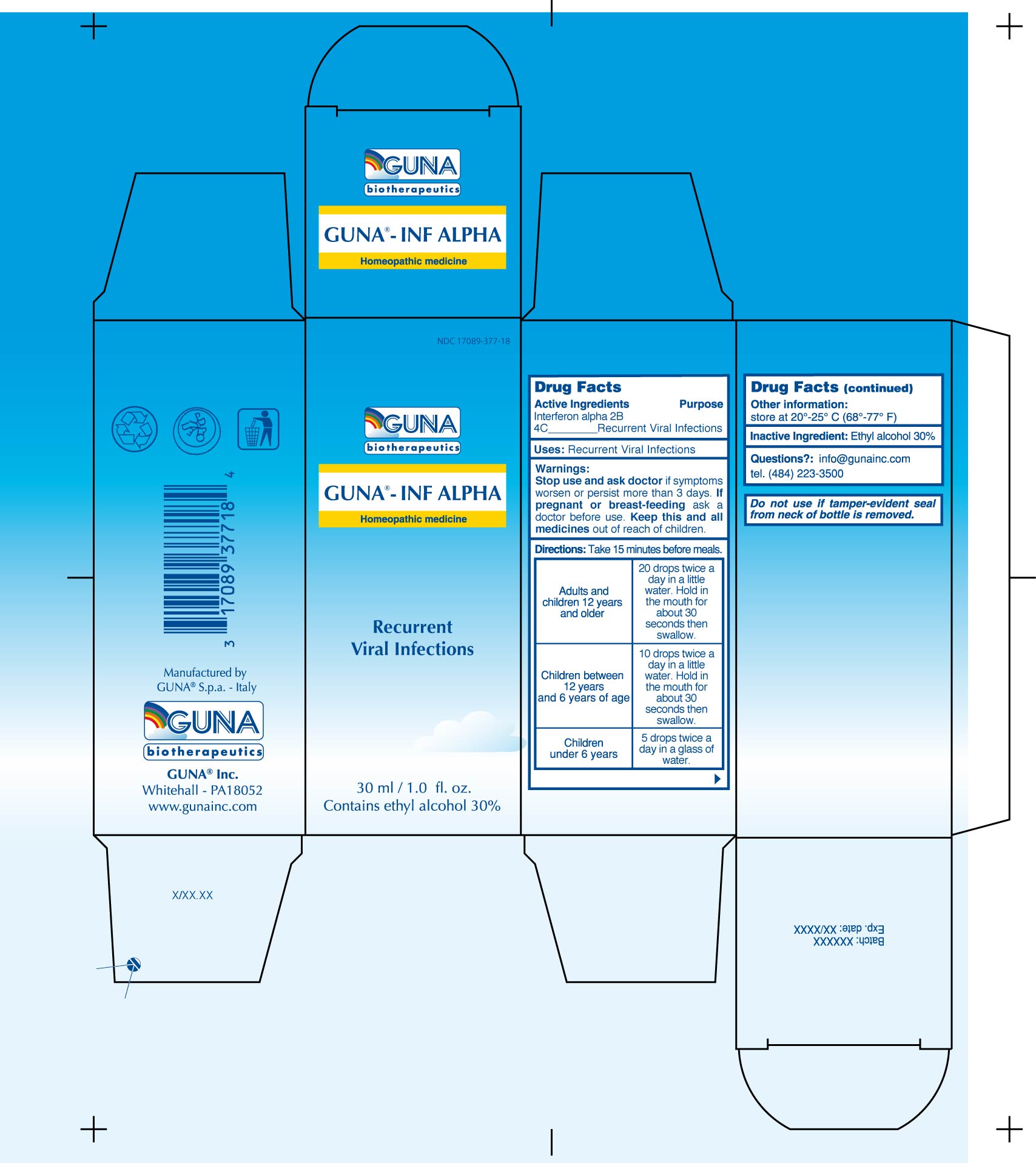
| GUNA-INF ALPHA
interferon alfa-2b solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved homeopathic | 06/17/2008 | 02/14/2011 | |
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Guna spa | 430538264 | manufacture | |