GLYTONE SOOTHE CALMING
-
hydrocortisone cream
Genesis Pharmaceuticals
----------
Soothe calming
Inactive Ingredients
Water (Aqua), Propylene glycol, Petrolatum, Cetearly Alcohol, Glycerin, PEg-40 Stearate.
Active Ingredients Purpose
Hydrocortisone 1% Anti pruritic(Anti-itch)
Warnings For external use only
When using this product
Keep out of eyes. Rinse with water to remove.
Do not use for the treatment of diaper rash, consult a doctor.
Stop use and ask a doctor if condition worsens or if symptoms persist for more than 7 days and occur again within a few days. Do not begin use of any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Uses
For temporary relief of itching associated with minor skin irritation and rashes. Other uses of this product should be only under the advice and supervision of a doctor.
Directions
Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
Children under 2 years of age: Do not use, consult a doctor.
Other Information
Store at room temperature
Do not freeze.
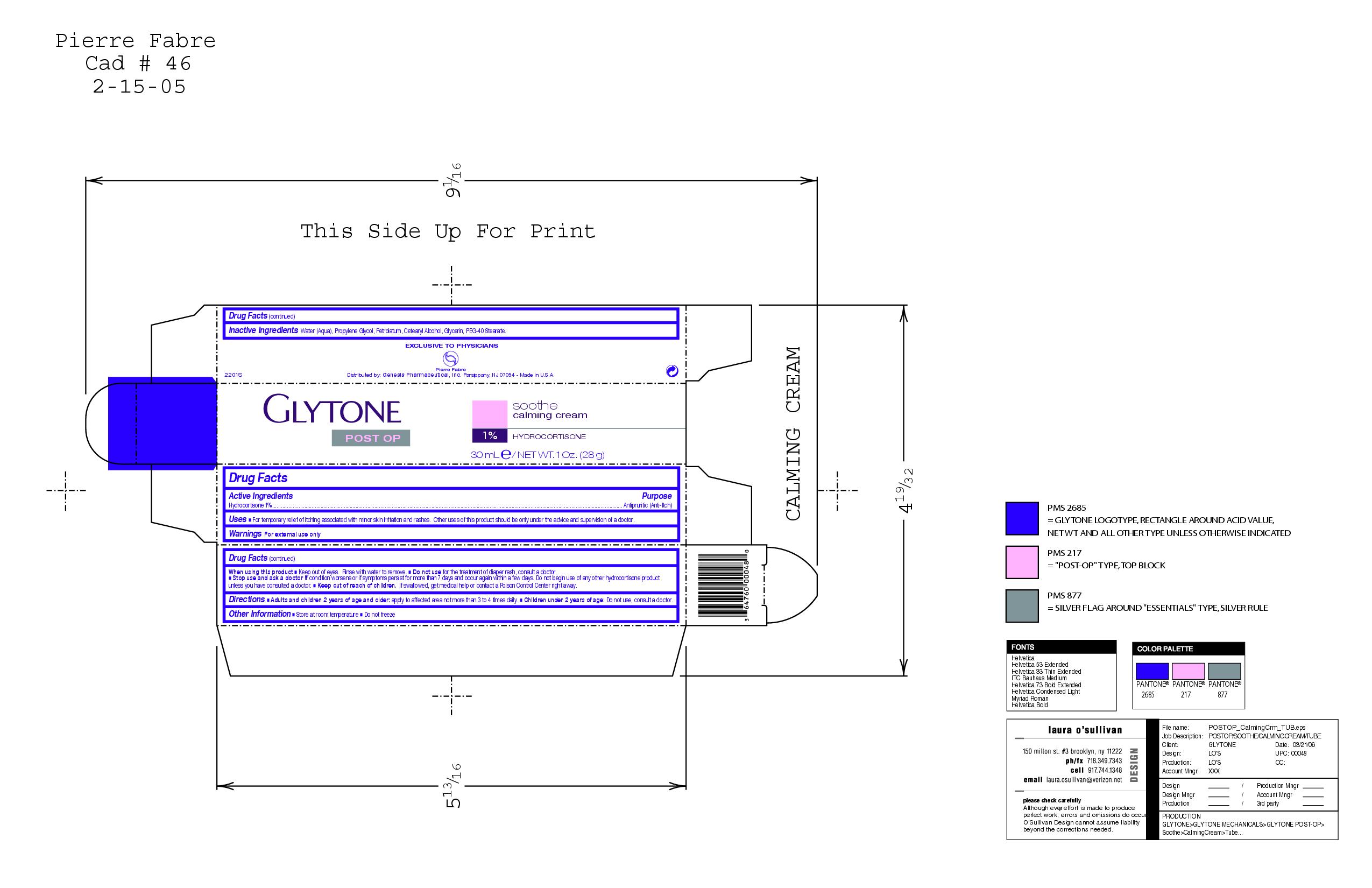
Glytone
Post Op
Soothe
Calming Cream
1% hydrocortisone
30 mL E/ NET WT. 1 Oz. (28 g)
Purpose Antipruritic (anti-itch)
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| GLYTONE SOOTHE CALMING
hydrocortisone cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part348 | 05/24/2010 | |
| Labeler - Genesis Pharmaceuticals (117196928) |