CHILDRENS CETIRIZINE HYDROCHLORIDE
-
cetirizine hydrochloride solution
Precision Dose Inc.
----------
CHILDREN'S CETIRIZINEHYDROCHLORIDE ORAL SOLUTION
5 mg/5 mL
Grape Flavor
For Hospital Use Only
Drug Facts
Active ingredient (in each 5 mL teaspoonful)
Cetirizine HCl 5 mg
Purpose
Antihistamine
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- if breast-feeding: not recommended
- if pregnant: ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- use as directed per healthcare professional
Other information
- store between 20° - 25°C (68° - 77°F)
- See individual label or shipper label for lot number and expiration date.
Inactive ingredients
acetic acid, artificial grape flavor, glycerin, methylparaben, natural banana flavor, propylene glycol, propylparaben, sodium acetate, sucrose, water
How Supplied
NDC 68094-720-62
5 mL per unit dose cup
Thirty (30) unit dose cups per shipper
Distributed By:
Perrigo Company
515 Eastern Avenue
Allegan, MI 49010
Packaged By:
Precision Dose, Inc.
722 Progressive Lane
S. Beloit, IL 61080
LI722
Rev. 12/08
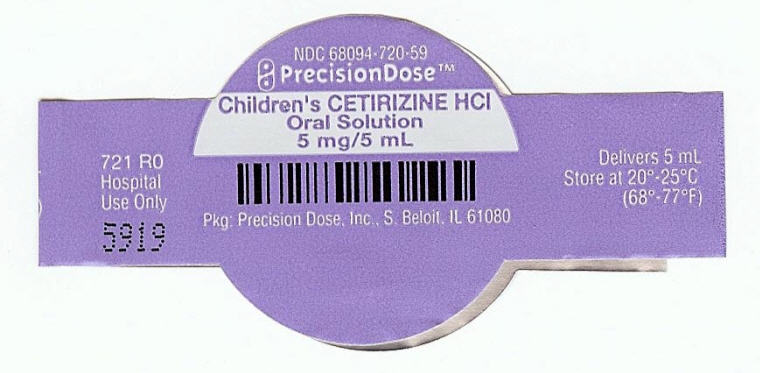
PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Cup Lid Label
NDC 68094-720-59
PrecisionDose™
Children's CETIRIZINE HCl
Oral Solution
5 mg/5 mL
Pkg: Precision Dose, Inc., S. Beloit, IL 61080
| CHILDRENS CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA090254 | 03/17/2009 | |
| Labeler - Precision Dose Inc. (035886746) |