aygestin (norethindrone acetate) tablet
[DURAMED PHARMACEUTICALS, INC.]
Rx only
Revised JULY 2005
51004242503
DESCRIPTION
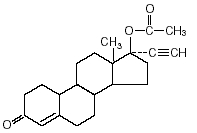
Aygestin (norethindrone acetate tablets, USP) - 5 mg oral tablets. Aygestin, (17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate), a synthetic, orally active progestin, is the acetic acid ester of norethindrone. It is a white, or creamy white, crystalline powder.
Aygestin Tablets contain the following inactive ingredients: lactose, magnesium stearate, and microcrystalline cellulose.
CLINICAL PHARMACOLOGY
Norethindrone acetate induces secretory changes in an estrogen-primed endometrium. It acts to inhibit the secretion of pituitary gonadotropins which, in turn, prevent follicular maturation and ovulation. On a weight basis, it is twice as potent as norethindrone.
INDICATIONS AND USAGE
Aygestin is indicated for the treatment of secondary amenorrhea, endometriosis, and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer.
CONTRAINDICATIONS
Known or suspected pregnancy.
Thrombophlebitis, thromboembolic disorders, cerebral apoplexy, or a past history of these conditions.
Markedly impaired liver function or liver disease.
Known or suspected carcinoma of the breast.
Undiagnosed vaginal bleeding.
Missed abortion.
As a diagnostic test for pregnancy.
Hypersensitivity to norethindrone acetate tablets.
WARNINGS
- Discontinue medication pending examination if there is a sudden partial or complete loss of vision or if there is sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, medication should be withdrawn. There have been reports of retinal vascular coincident with the use of progestins.
- Because of the occasional occurrence of thrombophlebitis and pulmonary embolism in patients taking progestogens, the physician should be alert to the earliest manifestations of the disease. Care should be used when prescribing progestins to a population that may be predisposed to thrombotic disorders (e.g., past history of thrombotic events, thrombophilia, obesity, cardiovascular disease, prolonged immobilization).
- Several reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. The risk of hypospadias, 5 to 8 per 1,000 male births in the general population, may be approximately doubled with exposure to these drugs. There are insufficient data to quantify the risk to exposed female fetuses, but insofar as some of these drugs induce mild virilization of the external genitalia of the female fetus, and because of the increased association of hypospadias in the male fetus, it is prudent to avoid the use of these drugs during the first trimester of pregnancy.
PRECAUTIONS
General Precautions
- The pretreatment physical examination should include special reference to breasts and pelvic organs, as well as a Papanicolaou smear.
- Because this drug may cause some degree of fluid retention, conditions which might be influenced by this factor, such as epilepsy, migraine, asthma, cardiac or renal dysfunctions, require careful observation.
- In cases of breakthrough bleeding, as in all cases of irregular bleeding per vagina, nonfunctional causes should be born in mind. In cases of undiagnosed vaginal bleeding, adequate diagnostic measures are indicated.
- Patients who have a history of psychic depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree.
- Any possible influence of prolonged proestogen therapy on pituitary, ovarian, adrenal, hepatic, or uterine functions awaits further study.
- Data suggest that progestin therapy may have adverse effects on lipid and carbohydrate metabolism. The choice of progestin, its dose, and its regimen may be important in minimizing these adverse effects, but these issues will require further study before they are clarified. Women with hyperlipidemias and/or diabetes should be monitored closely during progestin therapy.
- The age of the patient constitutes no absolute limiting factor, although treatment with progestogens may mask the onset of the climacteric.
- The pathologist should be advised of progestogen therapy when relevant specimens are submitted.
Information for the Patient
See text which appears at the end of this insert.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Some beagle dogs treated with medroxyprogesterone acetate developed mammary nodules. Although nodules occasionally appeared in control animals, they were intermittent in nature, whereas nodules in treated animals were larger and more numerous, and persisted. There is no general agreement as to whether the nodules are benign or malignant. Their significance with respect to humans has not been established.
Pregnancy Category X
Norethindrone acetate is contraindicated during pregnancy as it may cause fetal harm when administered to pregnant women. Several reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. Hypospadias occurs in about 5 to 8 per 1, 000 male births and is about doubled with exposure to these drugs. Some progestational drugs induce mild virilization of the external genitalia of female fetuses.
Nursing Mothers
Detectable amounts of progestogens have been identified in the milk of mothers receiving them. The effect of this on the nursing infant has not been determined.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
See “WARNINGS” for further information on Retinal vascular thrombosis, Thrombotic and thromboembolism events, and Use in pregnancy.
The following adverse reactions have been observed in women taking progestins:
Breakthrough bleeding.
Spotting.
Change in menstrual flow.
Amenorrhea.
Edema.
Changes in weight (decreases, increases).
Changes in cervical erosion and cervical secretions.
Cholestatic jaundice.
Rash (allergic) with and without pruritus.
Melasma or chloasma.
Mental depression.
Acne.
Breast enlargement/tenderness.
Headache/migraine.
Urticaria.
Abnormalities of liver tests (i.e., AST, ALT, Bilirubin).
Mood swings.
Nausea.
Insomnia.
Anaphylactic/anaphylactoid reactions.
Thrombotic and thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism, retinal vascular thrombosis, cerebral thrombosis and embolism).
Optic neuritis (which may lead to partial or complete loss of vision).
Progestins may alter the result of pregnanediol determinations. The following laboratory results may be altered by the concomitant use of estrogens with progestins:
Hepatic function.
Coagulation tests - increase in prothrombin, factors VII, VIII, IX, and X.
Increase in PBI, BEI, and a decrease in T3 uptake.
Reduced response to metyrapone test.
The following adverse reactions have been observed in patients receiving estrogen-progestogen combination drugs:
- Rise in blood pressure in susceptible individuals.
- Premenstrual-like syndrome.
- Changes in libido.
- Changes in appetite.
- Cystitis-like syndrome.
- Headache.
- Nervousness.
- Dizziness.
- Fatigue.
- Backache.
- Hirsutism.
- Loss of scalp hair.
- Erythema multiforme.
- Erythema nodosum.
- Hemorrhagic eruption.
- Itching.
In view of these observations, patients on progestogen therapy should be carefully observed.
DOSAGE AND ADMINISTRATION
Therapy with Aygestin® (norethindrone acetate tablets, USP) must be adapted to the specific indications and therapeutic response of the individual patient. This dosage schedule assumes the interval between menses to be 28 days.
Secondary amenorrhea, abnormal uterine bleeding due to hormonal imbalance in the absenceof organic pathology: 2.5 to 10 mg Aygestin may be given daily for 5 to 10 days during the second half of the theoretical menstrual cycle to produce an optimum secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen.
Progestin withdrawal bleeding usually occurs within three to seven days after discontinuing Aygestin therapy. Patients with a past history of recurrent episodes of abnormal uterine bleeding may benefit from planned menstrual cycling with Aygestin.
Endometriosis: Initial daily dosage of 5 mg Aygestin for two weeks. Dosage should be increased by 2.5 mg per day every two weeks until 15 mg per day of Aygestin is reached. Therapy may be held at this level for six to nine months or until annoying breakthrough bleeding demands temporary termination.
HOW SUPPLIED
Aygestin® (norethindrone acetate tablets, USP) are available as:
5 mg: White, oval, flat-faced, beveled edge, tablet scored on one side. Debossed with 5 Aygestin on the unscored side and stylized b /424 on the scored side.
Available in bottle of:
| 50 | NDC 51285-424-10 |
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature].
Dispense in a well-closed container.
INFORMATION FOR THE PATIENT
Your doctor has prescribed Aygestin (norethindrone acetate tablets, USP), a progestin, for you. Aygestin is similar to the progesterone hormones naturally produced by the body. Progestins are used to treat menstrual disorders and to test if the body is producing certain hormones.
WARNINGS
AYGESTIN TABLETS SHOULD NOT BE USED IN WOMEN WITH THE FOLLOWING CONDITIONS:
- - Known or suspected pregnancy.
- - History of blood clots in the legs, lungs, eyes, brain, or elsewhere, or a past history of these conditions.
- - Liver impairment or disease.
- - Known or suspected cancer of the breast.
- - Undiagnosed vaginal bleeding.
- - Hypersensitivity to Aygestin tablets.
The information below relates only to the risk to the unborn child associated with use of progestin during pregnancy, abnormal blood clotting, and eye abnormalities.
Risk to the Fetus
Aygestin tablets should not be used if you are pregnant. Norethindrone acetate is contraindicated during pregnancy as it may cause fetal harm when administered to pregnant women. There is an increased risk of minor birth defects in children whose mothers take this drug during the first 4 months of pregnancy. Several reports suggest an association between mothers who take these drugs in the first trimester of pregnancy and genital abnormalities in male and female babies. The risk to the male baby is the possibility of being born with a condition in which the opening of the penis is on the underside rather than the tip of the penis (hypospadias). Hypospadias occurs in about 5 to 8 per 1,000 male births and is about doubled with exposure to these drugs. There is not enough information to quantify the risk to exposed female fetuses, but enlargement of the clitoris and fusion of the labia may occur, although rarely. Therefore, avoid using the drug during the first trimester of pregnancy.
If you take Aygestin (norethindrone acetate tablets, USP) and later find you were pregnant when you took it, be sure to discuss this with your doctor as soon as possible.
Abnormal Blood Clotting
Use of progestational drugs has been associated with changes in the blood-clotting system. These changes allow the blood to clot more easily, possibly allowing clots to form in the bloodstream. If blood clots do form in your bloodstream, they can cut off the blood supply to vital organs, causing serious problems. These problems may include a stroke (by cutting off blood to part of the brain), a heart attack (by cutting off blood to part of the heart), a pulmonary embolus (by cutting off blood to part of the lungs), visual loss or blindness (by cutting off blood vessels in the eye), or other problems. Any of these condition may cause death or serious long-term disability. Call your doctor immediately if you suspect you have any of these conditions. He or she may advise you to stop using the drug.
Eye Abnormalities
Discontinue medication and call your physician immediately if you experience sudden partial or complete loss of vision, blurred vision, or sudden onset of bulging eyes, double vision, or migraine.
OTHER INFORMATION
For further information on the use, side effects and other risks associated with this product, ask your doctor. If you want more information, ask your doctor to show you the professional labeling. The professional labeling is also published in a book called the Physicians’ Desk Reference, which is available in bookstores and public libraries.
DURAMED PHARMACEUTICALS, INC.
A subsidiary of Barr Pharmaceuticals, Inc.
Pomona, New York 10970
Revised JULY 2005
BR-424
| Aygestin (norethindrone acetate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 06/2007DURAMED PHARMACEUTICALS, INC.