FERREX 28
-
ascorbic acid, folic acid, cyanocobalamin, iron, ferrous fumurate and succinic acid
Breckenridge Pharmaceutical, Inc.
----------
Ferrex™ 28ONCE-DAILY
Rx Only
DESCRIPTION
Ferrex™ 28 Tablets for oral administration to provide 28-day iron supplement therapy.
Each maroon film-coated tablet contains:
Vitamin C (Ascorbic Acid) .................................... 140 mg
Vitamin C
Ascorbic Acid (as Calcium Ascorbate)................... 60 mg
Threonic Acid (as Calcium Threonate) .................. 0.8 mg
Folic Acid, USP ........................................................ 1 mg
Vitamin B12 (Cyanocobalamin) ................................10 mcg
Iron (as Ferrous Asparto Glycinate) .......................... 70 mg
Iron (as Ferrous Fumarate)........................................ 81 mg
Succinic Acid.......................................................... 150 mg
Inactive Ingredients: Citric Acid, Croscarmellose Sodium, Crospovidone, Dicalcium Phosphate, FD&C Blue No. 1 Lake, FD&C Red No. 40 Lake, FD&C Yellow No. 6 Lake1, Hypromellose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Pharmaceutical Glaze, Polyethylene Glycol, Polyvinylpyrrolidone, Silica, Sodium Lauryl Sulfate, Soy Polysaccharide, Stearic Acid and Titanium Dioxide.
Allergen: Contains Soy.
Each blue film-coated tablet contains:
Succinic Acid..................................................... 150 mg
Inactive Ingredients: Croscarmellose Sodium, Crospovidone, Dicalcium Phosphate, FD&C Blue No. 1 Lake, Hypromellose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Silica, Soy Polysaccharide, Stearic Acid, Titanium Dioxide and Triacetin.
Allergen: Contains Soy.
- 1
- This product contains FD&C Yellow No. 6 Lake as a color additive.
INDICATIONS AND USAGE
For the nutritional supplementation of anemias responsive to oral iron, such as hypochromic anemia associated with pregnancy, chronic or acute blood loss, dietary restriction, metabolic disease and post-surgical convalescence.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. Hemochromatosis and hemosiderosis are contraindications to iron supplementation.
WARNINGS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS
General
Do not exceed recommended dose.
The type of anemia and the underlying cause or causes should be determined before taking Ferrex™ 28 Tablets. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. Pernicious anemia should be excluded before using this product since folic acid may mask the symptoms of pernicious anemia.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies on this product have not been performed in subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug supplementation.
If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, or health-care provider before using or continuing use.
ADVERSE REACTIONS
Adverse reactions with iron supplementation may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron supplementation are usually transient.
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
DOSAGE AND ADMINISTRATION
Usual adult dose is 1 tablet daily, or as directed by a physician. Ferrex™ 28 supplies a 28 day course of iron supplementation that consists of 21 maroon active tablets and 7 blue absorption period tablets. Take 1 maroon tablet daily for 21 days, followed by 1 blue tablet daily for 7 days. After 28 tablets have been taken, a new course may be started if prescribed.
HOW SUPPLIED
Ferrex™ 28 Tablets are available as 21 maroon active tablets debossed "B 586" and 7 blue absorption period tablets debossed "B 587". Supplied in packages of 28 tablets each containing 4 child-resistant blisters of 7 tablets. NDC 51991-588-28.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Storage and Handling
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP Controlled Room Temperature.
Protect from light and moisture.
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Rx Only
Manufactured by:
Contract Pharmacal Corp.
Hauppauge, NY 11788 USA
Distributed by:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
Iss. 05/09
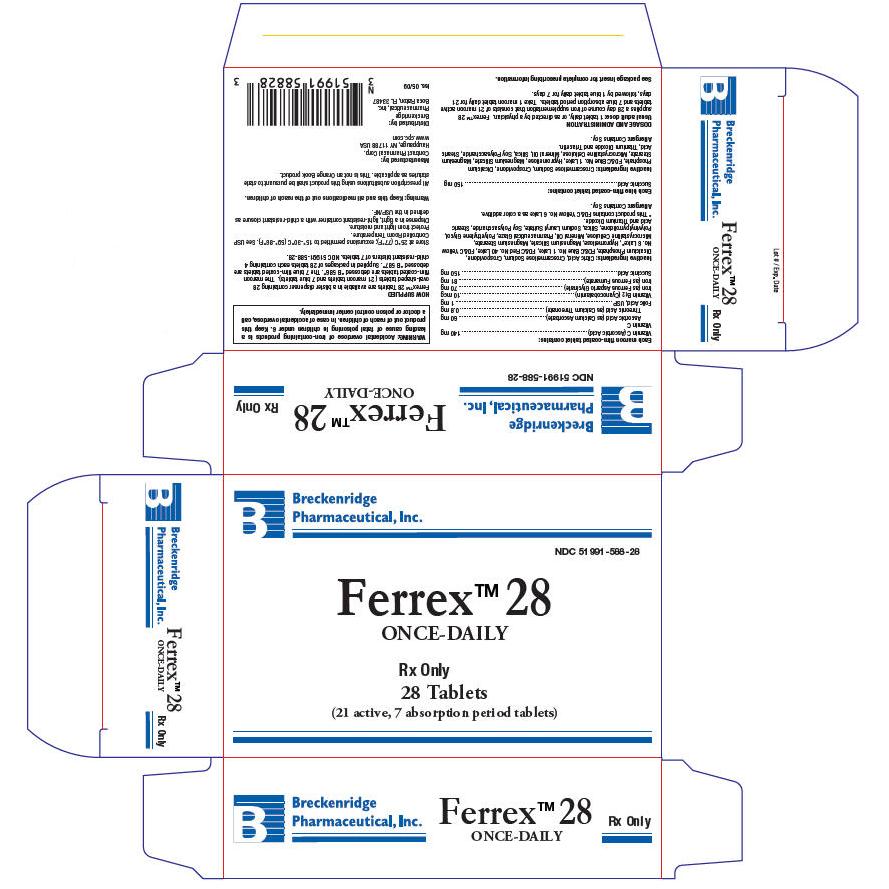
PRINCIPAL DISPLAY PANEL - Carton
Breckenridge
Pharmaceutical, Inc.
NDC 51991-588-28
Ferrex™ 28
ONCE-DAILY
Rx Only
28 Tablets
(21 active, 7 absorption period tablets)
| FERREX 28
ascorbic acid, folic acid, cyanocobalamin, iron, ferrous fumurate and succinic acid kit |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 06/26/2009 | 07/31/2011 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Contract Pharmacal Corporation | 057795122 | MANUFACTURE | |