HYOSCYAMINE SULFATE
-
hyoscyamine sulfate tablet
Prasco Laboratories
----------
Unknown Title11 DESCRIPTION
Each white, round uncoated compressed tablet for oral administration contains:
Hyoscyamine Sulfate............................................0.15 mg
Inactive ingredients include: lactose monohydrate, magnesium stearate, manitol starch (pregelatinized corn), and stearic acid.
Established Name: Hyoscyamine Sulfate
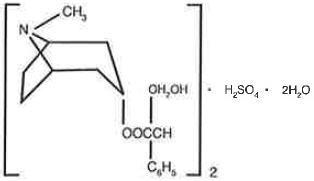
Chemical Name: Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo [3.2.1] oct-3-yl ester, [3(S)-endo]-, sulfate (2:1), dihydrate.
12 CLINICAL PHARMACOLOGY
Through its parasympatholytic action, hyoscyamine sulfate relaxes smooth muscle spasm resulting from parasympathetic stimulation. It inhibits gastrointestinal propulsive motility and decreases gastric acid secretion. It also controls excessive pharyngeal, tracheal and bronchial secretions. It is the I-isomer of atropine and therefore exhibits the same clinical effects as atropine. It is, however, approximately twice as active peripherally as atropine, since the latter is the reacemic (dl) form of hyoscyamine and d-hyoscyamine possesses only a very weak anticholinergic action. Since only one-half the atropine dose is required for I-hyoscyamine, it has only one-half the unwanted central effects of atropine.
1 INDICATIONS & USAGE
In the management of disorders of the lower urinary tract associated with hypermotility. Although specific therapy is often required to remove the underlying cause of spasm, Hyoscyamine Sulfate Tablets are offered as an antispasmodic agent which may be combined with other forms of therapy where indicated. Hyoscyamine Sulfate Tablets are effective as adjunctive therapy in the treatment of peptic ulcer and irritable bowel syndrome (irritable colon, spastic colon, mucous colitis), acute enter colitis and other functional gastrointestinal disorders.
Hyoscyamine Sulfate Tablets can also be used to control gastric secretion, visceral spasm and hypermotility in cystitis, pylorospasm and associated abdominal cramps. They may be used in functional intestinal disorders to reduce symptoms such as those seen in mild dysenteries and diverticulitis. They are indicated (along with appropriate analgesics) in symptomatic relief of biliary and renal colic.
4 CONTRAINDICATIONS
Glaucoma, obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive diseases of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis); paralytic ileum, intestinal atony of elderly or debilitated patients, unstable cardiovascular status, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis. Hypersensitivity to any of the ingredients.
WARNINGS
In the presence of high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy; in this instance, treatment would be inappropriate and possibly harmful. Like other anticholinergic agents, these products may produce drowsiness or blurred vision. In this event patients should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous tasks while taking this drug.
PRECAUTIONS
General:
Use with caution in patients with autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure and cardiac arrhythmia and hypertension. Investigate any tachycardia before giving any anticholinergic drugs since they may increase the heart rate. Use with caution in patients with hiatal hernia associated with reflux esophagitis.
17 INFORMATION FOR PATIENTS
Hyoscyamine Sulfate Tablets may cause drowsiness, dizziness or blurred vision; patients should observe caution before driving, using machinery or performing other tasks requiring mental alertness. Use of Hyoscyamine Sulfate Tablets may decrease sweating resulting in heat prostration, fever or heat stroke; febrile patients or those who may be exposed to elevated environmental temperatures should use caution. Prolonged use of Hyoscyamine Sulfate Tablets may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidasis, and discomfort.
7 DRUG INTERACTIONS
Additive adverse effects resulting from cholinergic blockade may occur when Hyoscyamine Sulfate Tablets are administered concomitantly with other antimuscarinics, amantadine, haloperidol, phenothiazines, monoamine oxidase (MAO) inhibitors, tricyclic antidepressants or some antihistamines. Antacids may interfere with the absorption of Hyoscyamine Sulfate tablets; take Hyoscyamine Sulfate Tablets before meals and antacids after meals.
1 CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
No long-term studies in animals have been performed to determine the carcinogenic, mutagenic or impairment of fertility potential of Hyoscyamine Sulfate Tablets.
Pregnancy: Pregnancy Category C.
Animal reproduction studies have not been conducted with this product. It is also not known whether this product can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hyoscyamine Sulfate Tablets should be taken by a pregnant woman only if clearly needed.
3 NURSING MOTHERS
Hyoscyamine Sulfate Tablets is excreted in human milk. Caution should be exercised when Hyoscyamine Sulfate Tablets are administered to a nursing woman.
6 ADVERSE REACTIONS
Adverse reactions may include dryness of the mouth, urinary hesitancy and retention; blurred vision; tachycardia; palpitations; mydriasis; cycloplegia; increased ocular tension; loss of taste; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; allergic reactions or drug idiosyncrasies; urticaria and other dermal manifestations; ataxia; speech disturbance; some degree of mental confusion and/or excitement (especially in elderly persons); and decreased sweating. Note: Slight dryness of the mouth is an indication that parasympathetic blockage is effective.
9 DRUG ABUSE AND DEPENDENCE
A dependence on the use of Hyoscyamine Sulfate Tablets has not been reported and due to the nature of its ingredients, abuse of Hyoscyamine Sulfate Tablets is not expected.
10 OVERDOSAGE
Symptoms of overdose include severe dryness of the mouth, nose, throat and hot dry flushed skin, hyperpyrexia (especially in children), difficulty or inability to swallow, difficult speech, dilated pupils and iris almost disappears, restlessness and garrulity indicating an irritability of the brain, marked tremors, convulsions, respiratory failure, death. In adults, symptoms or overdosage may begin in the range of ingestion of 0.6 to 1 mg with doses exceeding 1-2 eliciting more profound toxicity. Measures to be taken are immediate lavage of the stomach and injection of physostigmine 0.5 to 2 mg intravenously and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (tepid water sponge baths, hypothermic blanket). Excitement to a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 mL of a 2% solution) by rectal infusion
2 DOSAGE & ADMINISTRATION
Adults: One to two tablets four times daily or fewer if needed.
Children (12 and under):
Reduce dosage in proportion to age and weight.
16 HOW SUPPLIED
Bottles of 100 white, round tablets, imprinted with “402” on one side and the Prasco Logo on the other side. NDC 66993-402-02.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F) Protect from moisture.
Rx only
Manufactured For:
PRASCO LABORATORIES
Cincinnati, Ohio 45249
By:
Sovereign Pharmaceuticals, Ltd.
Fort Worth, Texas 76118
500094 Iss. 6/2002
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

| HYOSCYAMINE SULFATE
hyoscyamine sulfate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 05/01/2002 | 09/30/2009 | |
| Labeler - Prasco Laboratories (065969375) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sovereign Pharmaceuticals, Ltd. | 623168267 | MANUFACTURE | |